Advancing the science for patients with rare diseases
As part of Rare Disease Day, PhRMA released a new fact sheet “Great Strides Against Rare Diseases” highlighting the advancements made in 2015.
As part of Rare Disease Day, PhRMA released a new fact sheet “Great Strides Against Rare Diseases” highlighting the advancements made in 2015.
When you have a loved one with a rare disease, the importance of persevering to find new, innovative treatments takes on new meaning. This year on Rare Disease Day, I am excited to celebrate the advances we saw in treatments last year and the hope we have for continued progress in the years ahead.
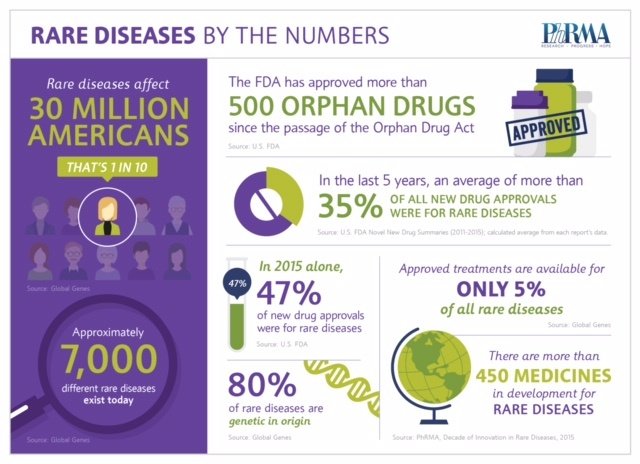
 Rare diseases are those that affect fewer than 200,000 people in the United States, but with more than 7,000 different rare diseases known today, nearly 1 in 10 Americans is living with a rare disease. Eighty percent of rare diseases – including the one impacting my family – are genetic in origin, making the rarity of each disease often unknown and treatment options especially limited. Today, approved treatments are available for just 5 percent of rare diseases.
Rare diseases are those that affect fewer than 200,000 people in the United States, but with more than 7,000 different rare diseases known today, nearly 1 in 10 Americans is living with a rare disease. Eighty percent of rare diseases – including the one impacting my family – are genetic in origin, making the rarity of each disease often unknown and treatment options especially limited. Today, approved treatments are available for just 5 percent of rare diseases.
But the hope for new treatments and cures has never been greater, with recent advances delivering on the promise of the science for patients. Today, PhRMA released a new fact sheet “Great Strides Against Rare Diseases” highlighting the advancements made for patients in the last year.
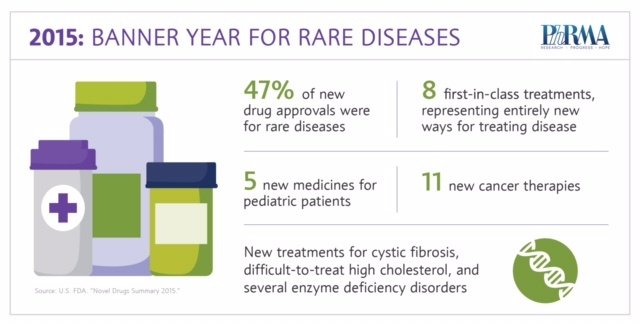
2015 was a banner year for advances in rare disease treatments:
 The fact sheet explores 2015 treatment advances in rare diseases, including new therapeutic options for patients with multiple myeloma and cystic fibrosis, as well as new treatments for several rare diseases affecting pediatric patients.
The fact sheet explores 2015 treatment advances in rare diseases, including new therapeutic options for patients with multiple myeloma and cystic fibrosis, as well as new treatments for several rare diseases affecting pediatric patients.
Together as industry, patients and advocates, we must continue to work together to foster research and development for these complex and challenging disease areas and maintain incentives critical to bringing new medicines to patients. The biopharmaceutical industry is committed to finding new treatments and cures for patients with rare diseases, and with 450 medicines in development, patients have more hope than ever before.