Driving innovation through collaboration
A new report examines the changing landscape of biopharmaceutical research, as R&D stakeholders are coming together to tackle vexing health challenges.

Driving innovation through collaboration.
A new report examines the changing landscape of biopharmaceutical research, as R&D stakeholders are coming together to tackle vexing health challenges.

Driving innovation through collaboration.
A new report from Deloitte examines the changing landscape of biopharmaceutical research, as stakeholders across the R&D ecosystem are increasingly coming together to tackle sciences’ most vexing health challenges.
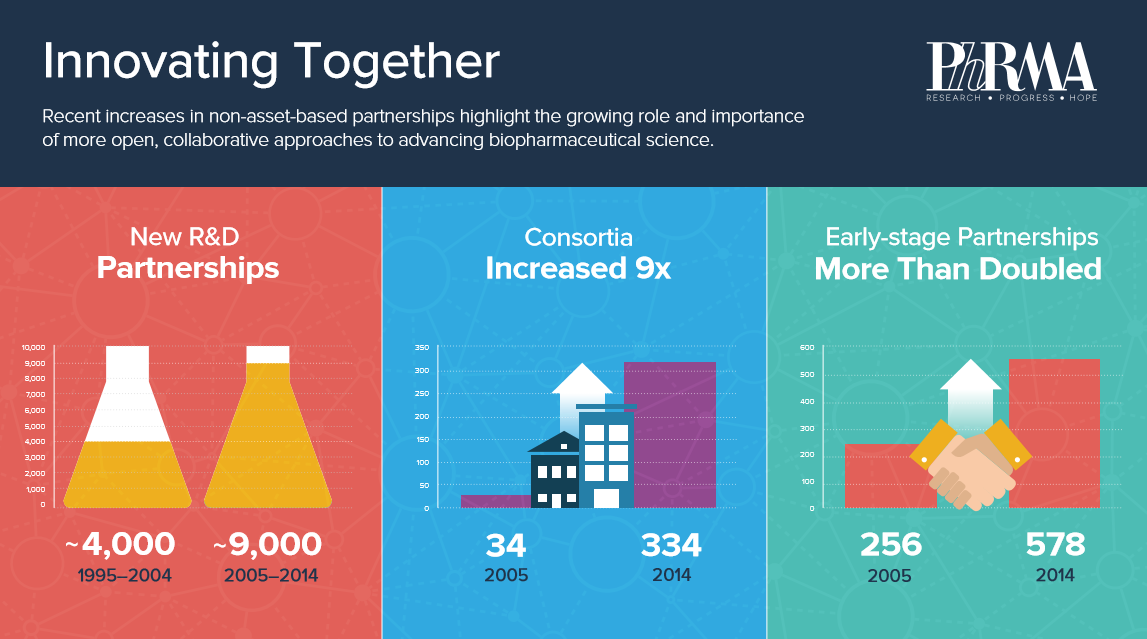
The new research illustrates there has been a significant shift in recent years from traditional, asset-based or transactional agreements to more open, collaborative approaches for conducting biopharmaceutical research.
Deloitte compiled and analyzed a database of thousands of partnerships initiated between 1995 and 2014. In that time the number of research and development partnerships grew from about 4,000 to approximately 9,000.
Not only was the number of partnerships changing, but also the type. Between 2005 and 2014 the number of early stage partnerships, taking place in the discovery or preclinical phases, more than doubled (from 256 to 578) and the number of consortia, with three or more partners, grew more than nine fold, from 34 to 334.
The report also includes examples that illustrate the breadth and variety of partnerships underway. Researchers are working together to address some of the most complex disease areas, where there is a serious and unmet need, including Alzheimer’s disease, various forms of cancer, and rare diseases among many others.
These data reflect both the growing complexity of the underlying science and the diseases researchers are tackling as well as recognition of the value of collaboration between and among various members of the R&D ecosystem to advance the science and bring benefits to patients and society.
Stakeholders from across the ecosystem, including biopharma companies, academia, federal researchers, patient groups, providers, and others, are working together to advance the science by leveraging each other’s strengths. Together, these partnerships are uncovering important learnings about the underlying drivers of many of the most devastating and complex diseases, driving innovation for patients.
Click here to read the entire report.