Last year was an exceptionally strong year for biopharmaceutical innovation as the U.S. Food and Drug Administration (FDA) approved 45 new medicines – the highest number of approvals in almost two decades - giving patients even greater hope for the future.
According to the FDA’s Center for Drug Evaluation and Research’s (CDER) fifth annual Novel New Drugs Summary, 45 new medicines were approved in 2015. More than a third (36 percent) of the new medicines approved were first-in-class treatment options – offering a completely new way to treat diseases – and nearly half (47 percent) were for rare conditions. An example of type of innovation that occurred is the novel oncolytic virus therapy for the treatment of melanoma, the most common cancer type in the United States. Other innovative medicines approved include a new generation of cancer treatments, including immunotherapies and personalized medicines; new treatments for diabetes, asthma and irritable bowel syndrome; and a new class of cholesterol-lowering medicines that address a significant unmet medical need for patients who couldn’t be treated with statin therapies.
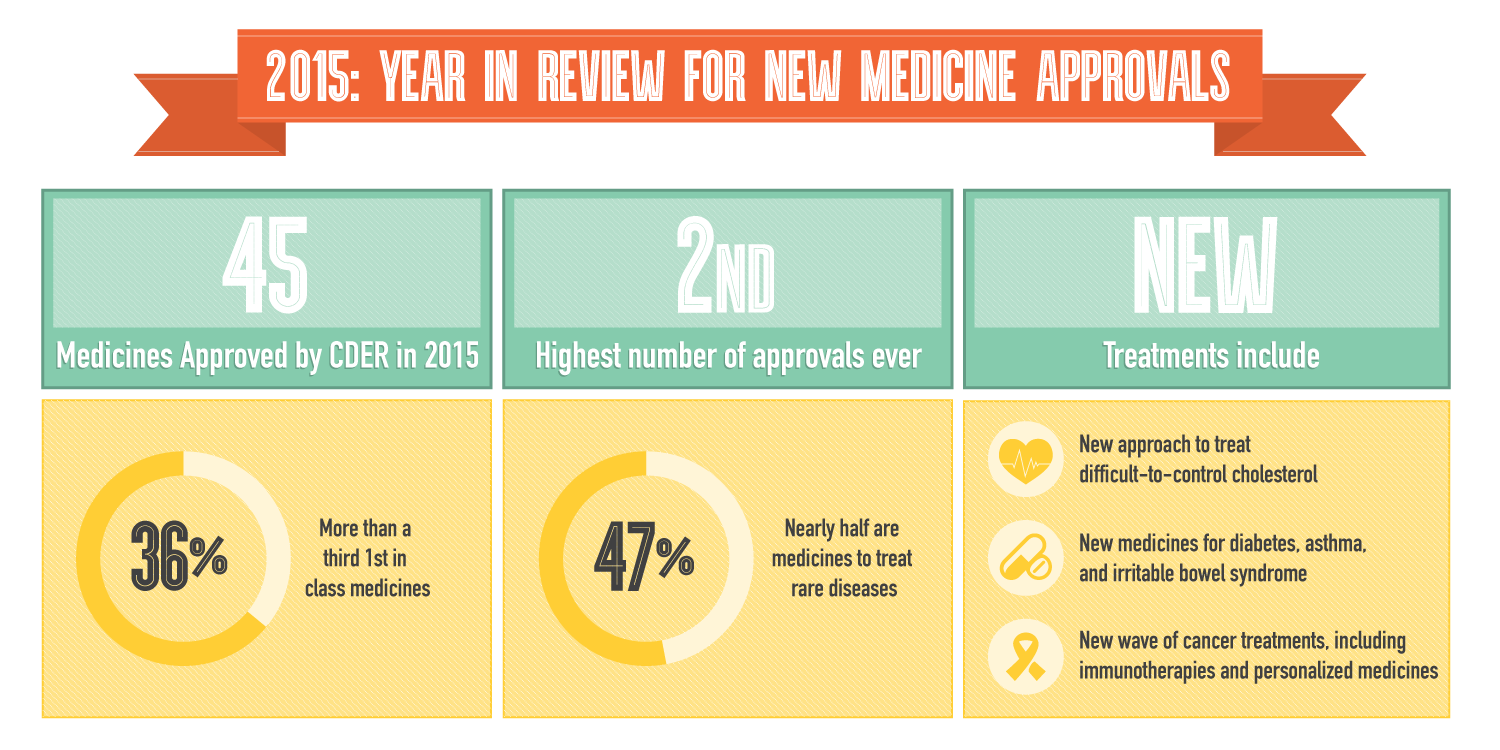 Last year’s remarkable number of approvals is in part due to the industry’s efforts to pursue new, collaborative approaches to streamline the research and development process. While R&D remains a challenge, America’s biopharmaceutical companies are committed to researching new treatments to help patients live longer, healthier lives.
Last year’s remarkable number of approvals is in part due to the industry’s efforts to pursue new, collaborative approaches to streamline the research and development process. While R&D remains a challenge, America’s biopharmaceutical companies are committed to researching new treatments to help patients live longer, healthier lives.
Learn more on what made 2015 a great year for new medicine approvals at www.innovation.org.
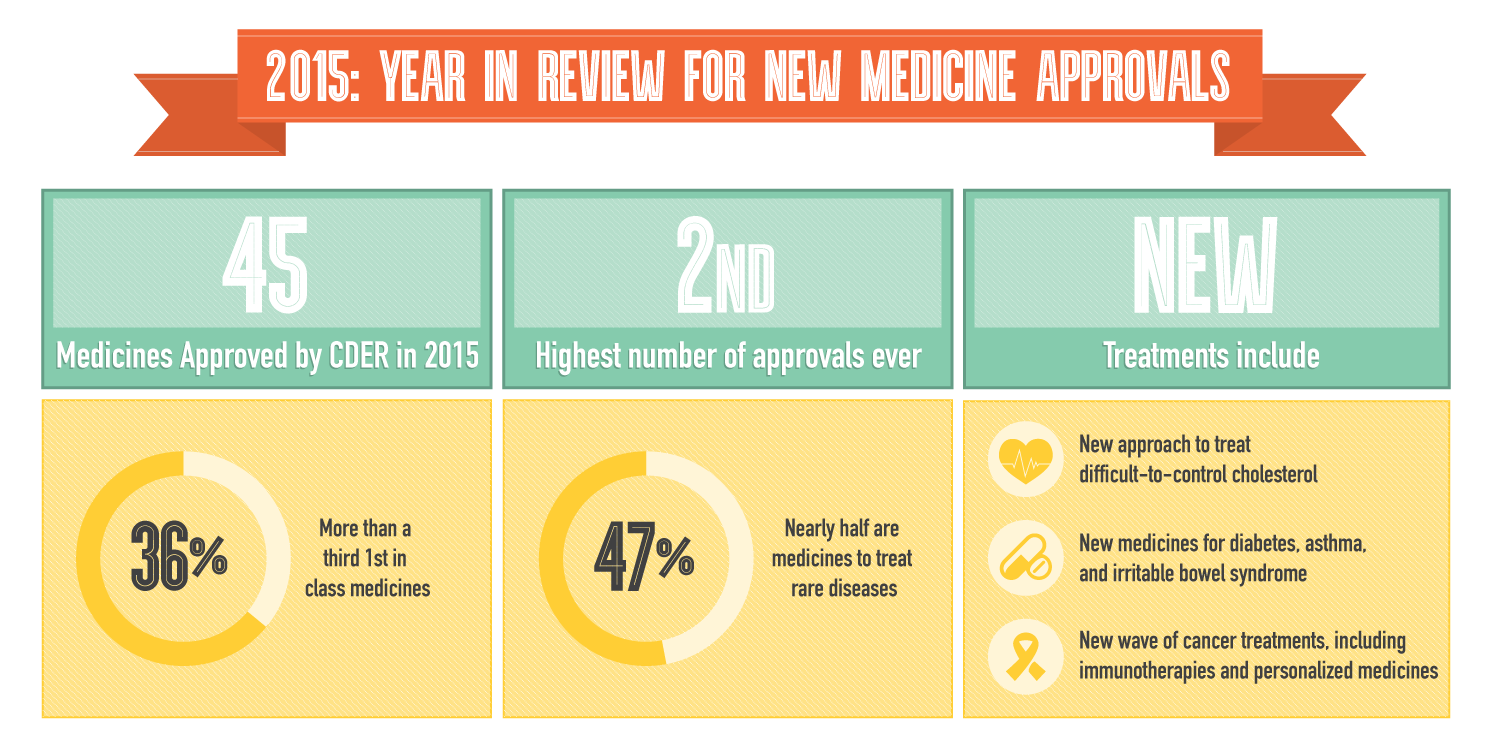


 Last year’s remarkable number of approvals is in part due to the industry’s efforts to pursue new, collaborative approaches to streamline the research and development process. While R&D remains a challenge, America’s biopharmaceutical companies are committed to researching new treatments to help patients live longer, healthier lives.
Last year’s remarkable number of approvals is in part due to the industry’s efforts to pursue new, collaborative approaches to streamline the research and development process. While R&D remains a challenge, America’s biopharmaceutical companies are committed to researching new treatments to help patients live longer, healthier lives.