In case you missed it, a special report, “Accelerating the Delivery of Patient-Centered, High-Quality Cancer Care,” published online yesterday in the American Association for Cancer Research (AACR) journal Clinical Cancer Research. Authored by Turning the Tide Against Cancer co-conveners, the report proposes four key policy recommendations to further the national dialogue on accelerating the delivery of patient-centered, high-quality cancer research and care.
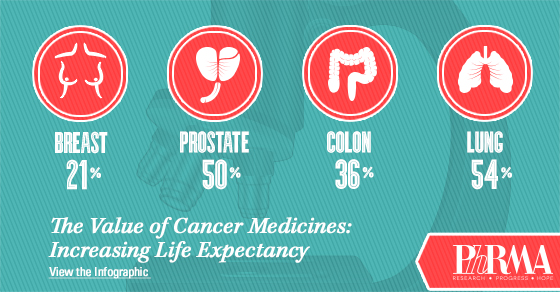 Notably, the report acknowledges that as the oncology community seeks to ensure innovation in cancer research and care continues, it is critical that stakeholders focus their attention on the value cancer research and care provides to patients and society. And the value is evident – in the U.S., cancer death rates are down nearly 22 percent from their peak, and the 5-year survival rate is now 68 percent.
Notably, the report acknowledges that as the oncology community seeks to ensure innovation in cancer research and care continues, it is critical that stakeholders focus their attention on the value cancer research and care provides to patients and society. And the value is evident – in the U.S., cancer death rates are down nearly 22 percent from their peak, and the 5-year survival rate is now 68 percent.
Key recommendations include:
- Support the U.S. Food and Drug Administration’s efforts to modernize its framework for bringing new medicines to patients, through facilitating and implementing innovative approaches to drug development and regulatory review.
- Ensure that cancer clinical pathways or similar decision-support tools are transparent; developed through a physician-driven process that includes patient input; and meet minimum standards for clinical appropriateness, timeliness, and patient centeredness.
- Support oncology decision support tools that are timely, clinically appropriate, and patient centered.
- Build on existing efforts to convene a multistakeholder committee and develop a report on ways to define and measure value in oncology care, taking into account many of the complex dynamics associated with measuring value, including the interests and needs of patients, as well as the importance of committed and ongoing support for innovative research.
Read more about the progress America’s biopharmaceutical companies have made in cancer research here.
Learn more about PhRMA’s position on payment reforms here.
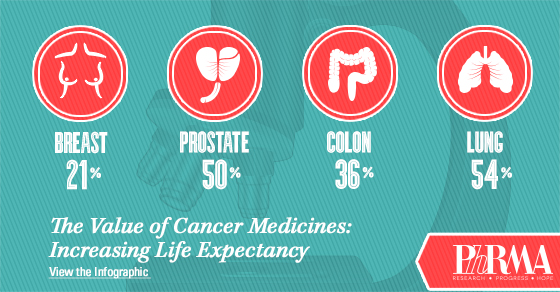


 Notably, the report acknowledges that as the oncology community seeks to ensure innovation in cancer research and care continues, it is critical that stakeholders focus their attention on the value cancer research and care provides to patients and society. And the value is evident – in the U.S., cancer death rates are down nearly 22 percent from their peak, and the 5-year survival rate is now 68 percent.
Notably, the report acknowledges that as the oncology community seeks to ensure innovation in cancer research and care continues, it is critical that stakeholders focus their attention on the value cancer research and care provides to patients and society. And the value is evident – in the U.S., cancer death rates are down nearly 22 percent from their peak, and the 5-year survival rate is now 68 percent.