 Since the implementation of Medicare Part D, we’ve seen numerous signs of success when it comes to how the program works and how it helps seniors and individuals with disabilities live longer, healthier lives. Take, for example, the fact that gaining Part D coverage was tied to an 8 percent decrease in hospital admissions. Or that close to 200,000 beneficiaries have lived at least one year longer following the implementation of Part D in 2006. And there are more medicines in development for older Americans than before Part D was implemented.
Since the implementation of Medicare Part D, we’ve seen numerous signs of success when it comes to how the program works and how it helps seniors and individuals with disabilities live longer, healthier lives. Take, for example, the fact that gaining Part D coverage was tied to an 8 percent decrease in hospital admissions. Or that close to 200,000 beneficiaries have lived at least one year longer following the implementation of Part D in 2006. And there are more medicines in development for older Americans than before Part D was implemented.
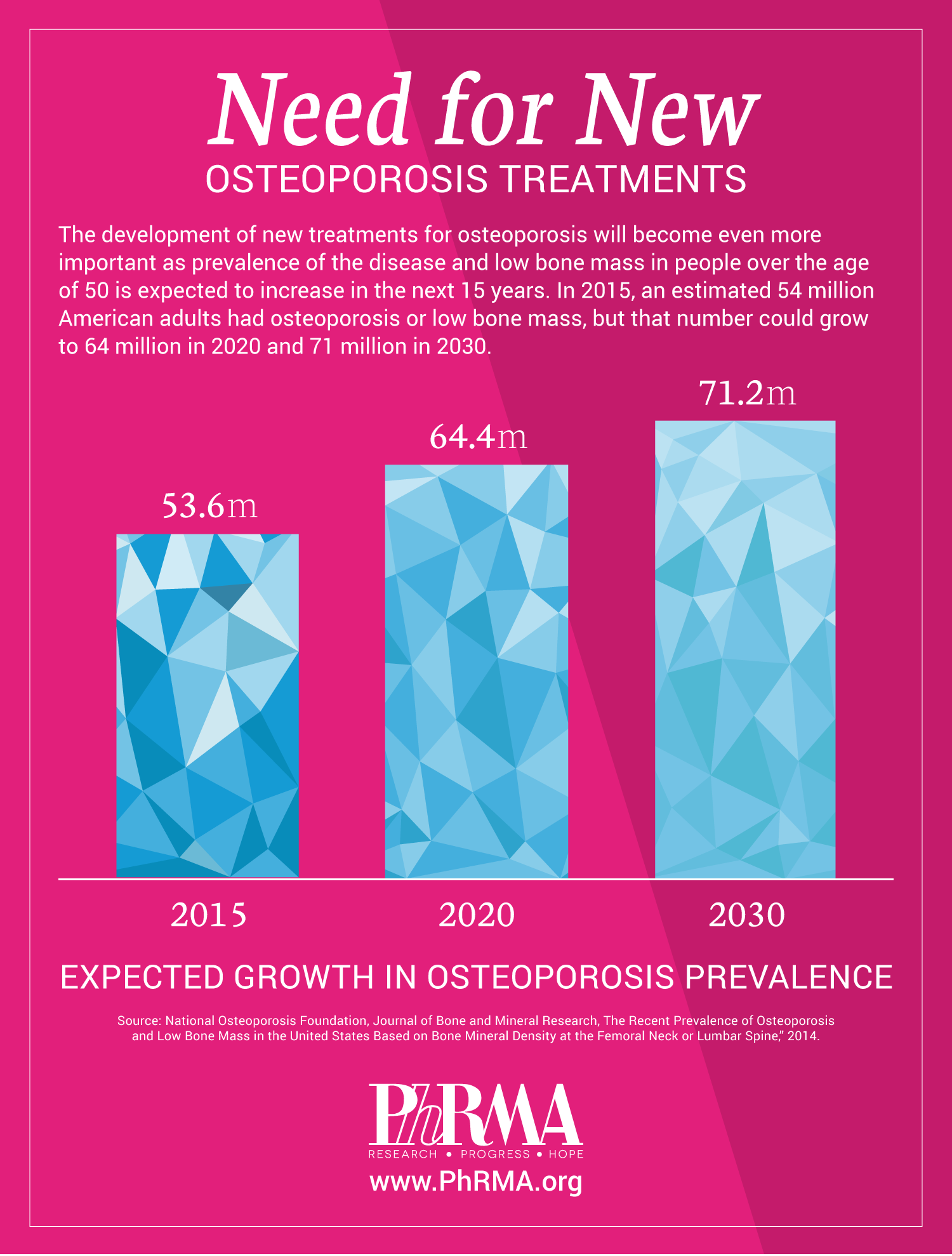 For osteoporosis, a disease that affects millions of Americans, there are currently nine medicines in clinical trials or undergoing review by the U.S. Food and Drug Administration.
For osteoporosis, a disease that affects millions of Americans, there are currently nine medicines in clinical trials or undergoing review by the U.S. Food and Drug Administration.
That’s according to a new report from PhRMA and the National Osteoporosis Foundation, “Medicines in Development for Osteoporosis.” The report also found that there are 34 clinical trials studying osteoporosis treatments across the United States, and America’s biopharmaceutical research companies are continuing to work to advance the treatment of this disease.
For Americans 50 or older, research shows that 50 percent of women and a quarter of men will suffer from osteoporosis. As America’s aging population grows, advancements in diagnosis and treatments are needed.
Each step toward a new treatment or new cure provides hope for not only the patients, but also their families, as they learn to live with osteoporosis. That is progress, and it is progress we should be excited about.
Learn more in the new report here:
http://phrma.org/sites/default/files/pdf/medicines-in-development-report-osteoporosis.pdf
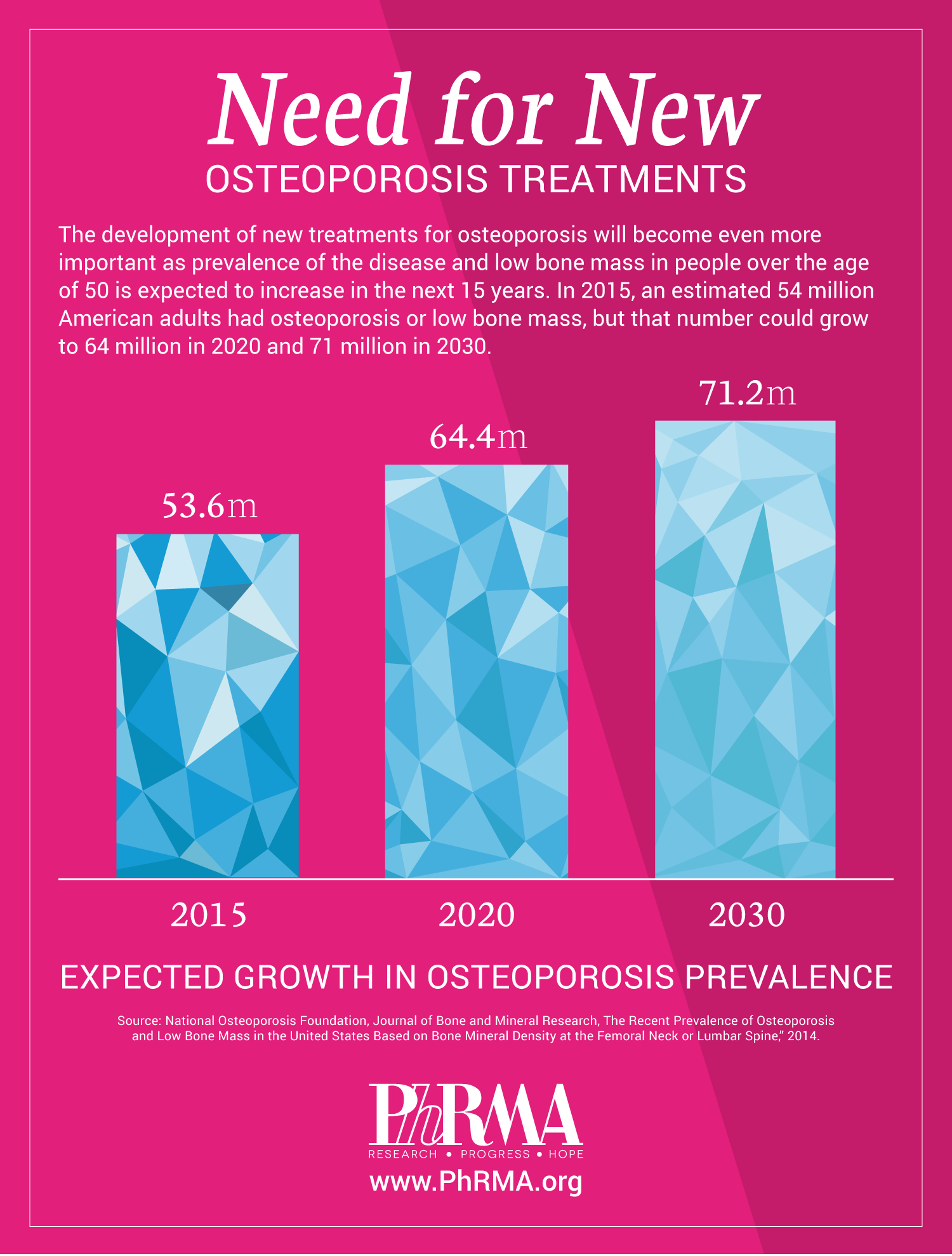


 Since the implementation of Medicare Part D, we’ve seen numerous signs of
Since the implementation of Medicare Part D, we’ve seen numerous signs of  For osteoporosis, a disease that affects millions of Americans, there are currently nine medicines in clinical trials or undergoing review by the U.S. Food and Drug Administration.
For osteoporosis, a disease that affects millions of Americans, there are currently nine medicines in clinical trials or undergoing review by the U.S. Food and Drug Administration.