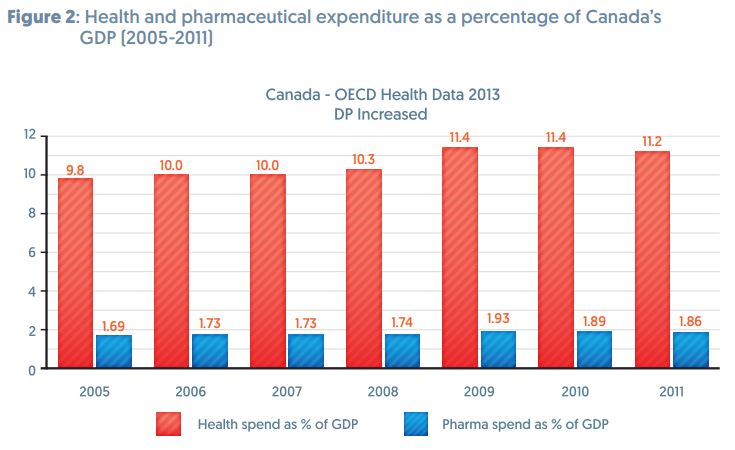
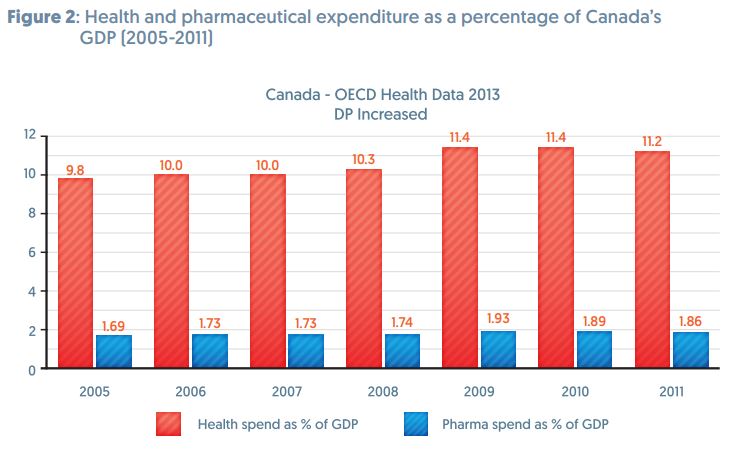
There has been much discussion about the effects of adding 12 years of data protection for biologics in the TPP. However, fears that this could add to the cost of healthcare seem to be unfounded.
A new paper by the Geneva Network using OECD data analyzed what happened to public spending on medicines when Canada and Japan increased their terms for regulatory data protection (RDP).
The Trans Pacific Partnership (TPP) is negotiating longer terms for RDP. In recent years both Canada and Japan increased their terms. In 2006 Canada changed its regulations that increased RDP from 0 to 8 years. Since then pharmaceutical spending as a percentage of total healthcare spending has actually decreased.
In Japan, RDP was increased from 6 years to 8 years in 2008. From 2005-2010 pharmaceutical spend as a percent of GDP remained essentially flat while overall health spend as a 5 of GDP increased by more than 10%.
The report does state that “there could be many explanations for these results….However, the evidence suggest that those concerned about access to medicines and the financial stability of public healthcare systems should focus their attention on policies other than RDP for medicines.”