While Canada may have prevailed in the recent North American Free Trade Agreement (NAFTA) tribunal case against Eli Lilly, the promise utility doctrine continues to harm their economy. And, unfortunately, that message likely will be lost. The Canadian government and its anti-intellectual property allies are claiming that the NAFTA tribunal endorsed the patent utility doctrine, striking a clear blow to Lilly’s contention that revocation of their patents was unjustified. In fact, the NAFTA tribunal made no such claim. The three-judge panel instead only ruled that Lilly had not met the threshold requirement of showing a dramatic shift in policy. As such, the tribunal did not need to consider the inconsistency of Canada’s patent utility doctrine with its international obligations, let alone whether the policy is good for Canada.
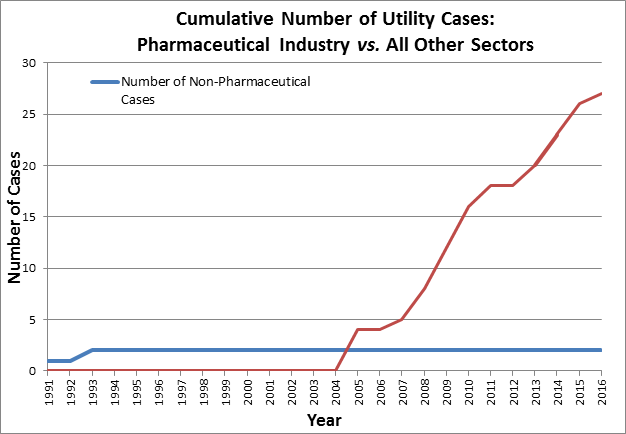
Prior to 2005, Canada never revoked a patent for a utility claim. In the intervening 12 years, 28 cases were decided based on the patent utility doctrine, resulting in the revocation of 25 patents on 21 separate medicines. No other country has come close to stripping intellectual property rights from the pharmaceutical industry in this manner. This, at a time when Canada is actively working to grow its innovative economy and spur the domestic commercialization of new inventions. This doctrine continues to thwart those efforts and has contributed to a 21 percent reduction in the number of clinical trials conducted in Canada. Future losses in pharmaceutical R&D could be even greater.
But, the Canadian Government has a choice. Through a quick legislative fix, Canadian courts would be required to live up to global standards of patentability, which purposely set a low bar for demonstrating utility. This is the same standard implemented in the United States, Europe and Japan – the standard that has led to robust innovative pharmaceutical sectors in each of those economies. Canada can chose to continue to isolate itself from the most-R&D intensive sector in the global economy or it can pass a legislative fix and attract some of the more than $100 billion invested into pharmaceutical innovation every year. The choice for Canada and its economy should be easy.




