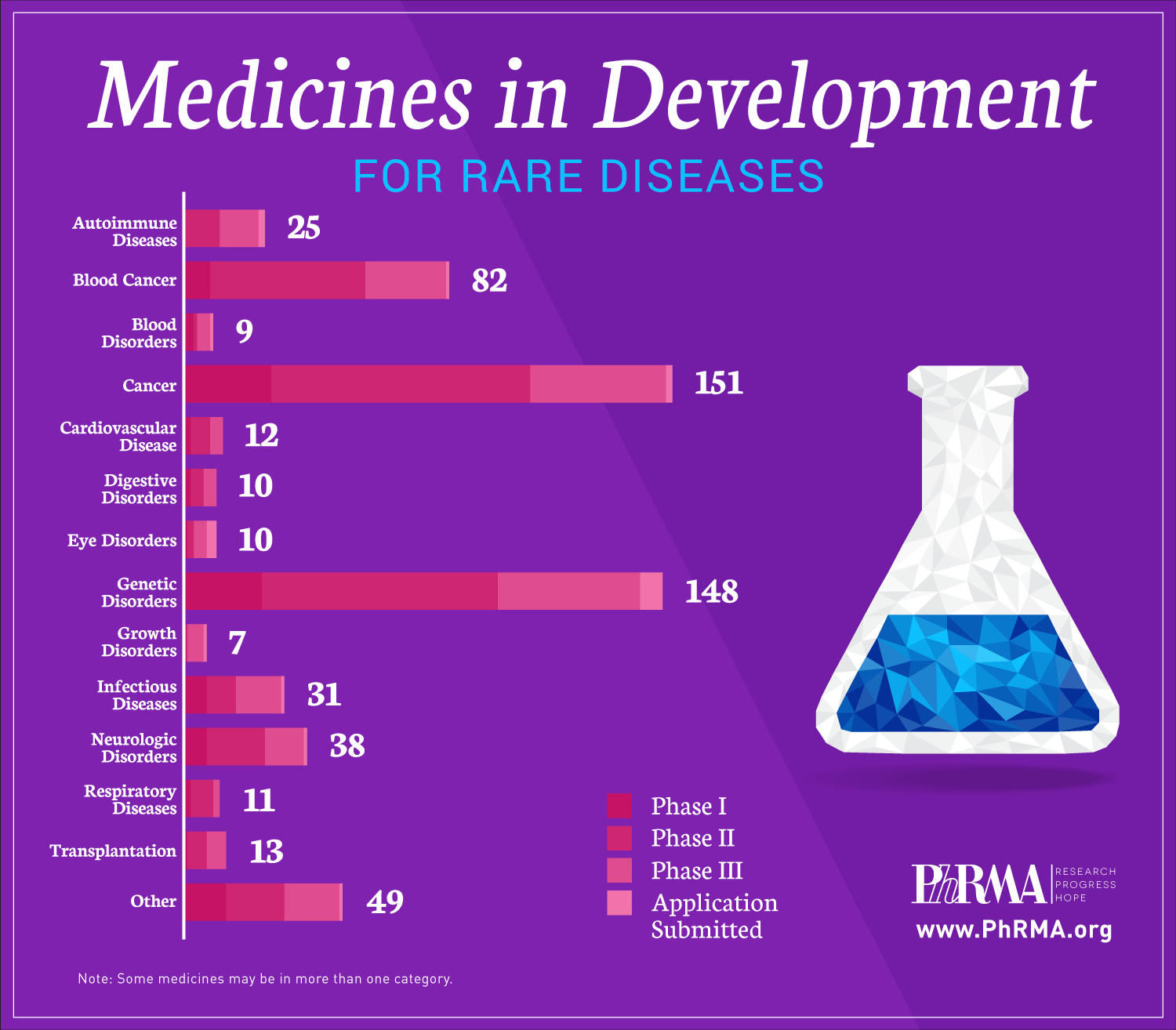
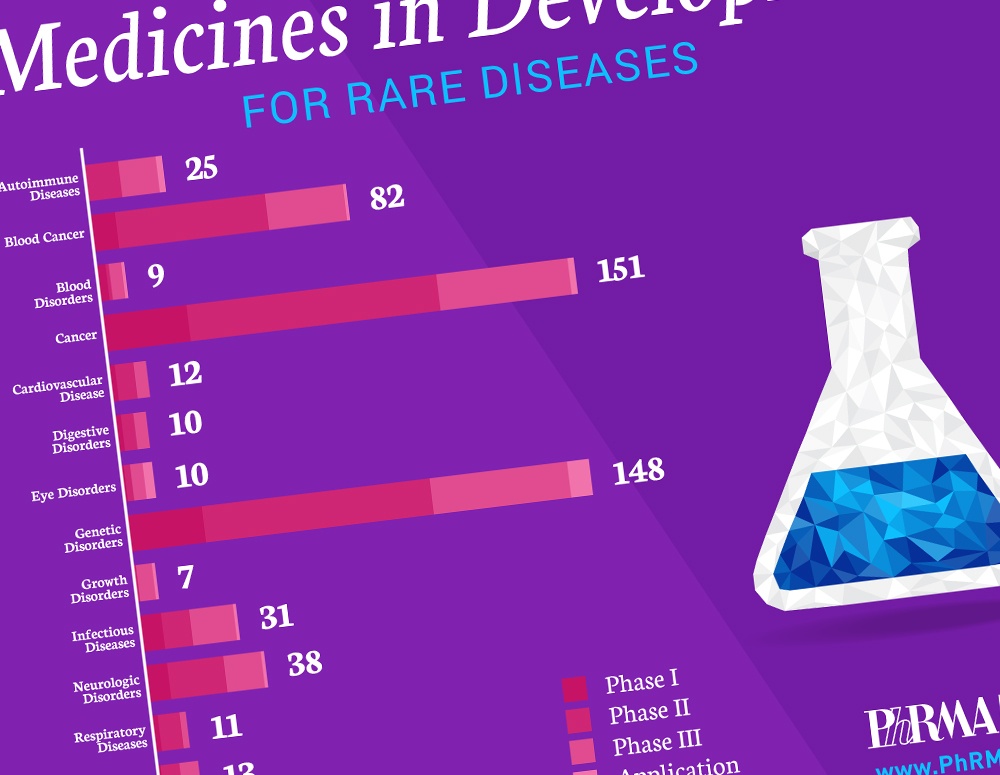
Today, there are 7,000 known rare diseases, or diseases that affect fewer than 200,000 people in the United States. But those suffering from the impact of these diseases have more hope today because of advances in science that promise brighter futures for American patients and families.
A new report, “Medicines in Development for Rare Diseases,” offers insight into the more than 560 novel treatments currently in the biopharmaceutical pipeline for the 30 million Americans currently living with a rare disease that have few or no treatment options, including patients with multiple myeloma, cystic fibrosis, amyotrophic lateral sclerosis (ALS) and enzyme deficiency disorders.
These medicines in development utilize new approaches for targeting diseases and include:
- 151 for rare cancers and 82 for rare blood cancers;
- 148 for genetic disorders, including cystic fibrosis and spinal muscular atrophy;
- 38 for neurological disorders, including ALS and seizures;
- 31 for infectious diseases, including rare bacterial infections and hepatitis; and
- 25 for autoimmune diseases, including systemic sclerosis and juvenile arthritis.
The neurological disorder ALS, also known as Lou Gehrig’s Disease, is just one of the numerous rare diseases with no cure. But new therapies in development are a step toward helping patients and their families manage this disease, as well as others in need of treatment.
Biopharmaceutical companies continue to pioneer the development of novel treatments for patients living with rare diseases. But this effort is a collaborative one. With the efforts of biopharmaceutical companies, disease groups like The ALS Association and patients alike, those affected by rare diseases can make progress toward healthier futures.
View the full report here.