Both payers and providers are interested in additional information from biopharmaceutical companies about prescription medicines – including information about pipeline medicines and unapproved uses of approved medicines. This finding is from a recent survey of payer executives and specialty physicians conducted by Health Strategies Group. The survey underscores that increased access to more complete information about medicines would help payers make better-informed coverage decisions and allow providers to make better prescribing decisions for patients.
Key findings of the survey:
- 80 percent or more of payers and providers are interested in receiving more data from biopharmaceutical companies regarding products undergoing clinical trials, as well as information on approved medicines, for both U.S. Food and Drug Administration-approved (FDA) uses and unapproved uses of approved medicines.
- The majority of payers and providers are already actively seeking this information, highlighting a demonstrated desire for this data.
- Payers and providers recognize and agree that there is a need for guardrails to ensure communications are scientifically sound and support appropriate, evidence-based decision-making.
- Payers continue to place strong emphasis on FDA approval in their decision-making.
- Providers report access to additional data about unapproved uses would increase patient referrals to clinical trials that may support labeling changes.
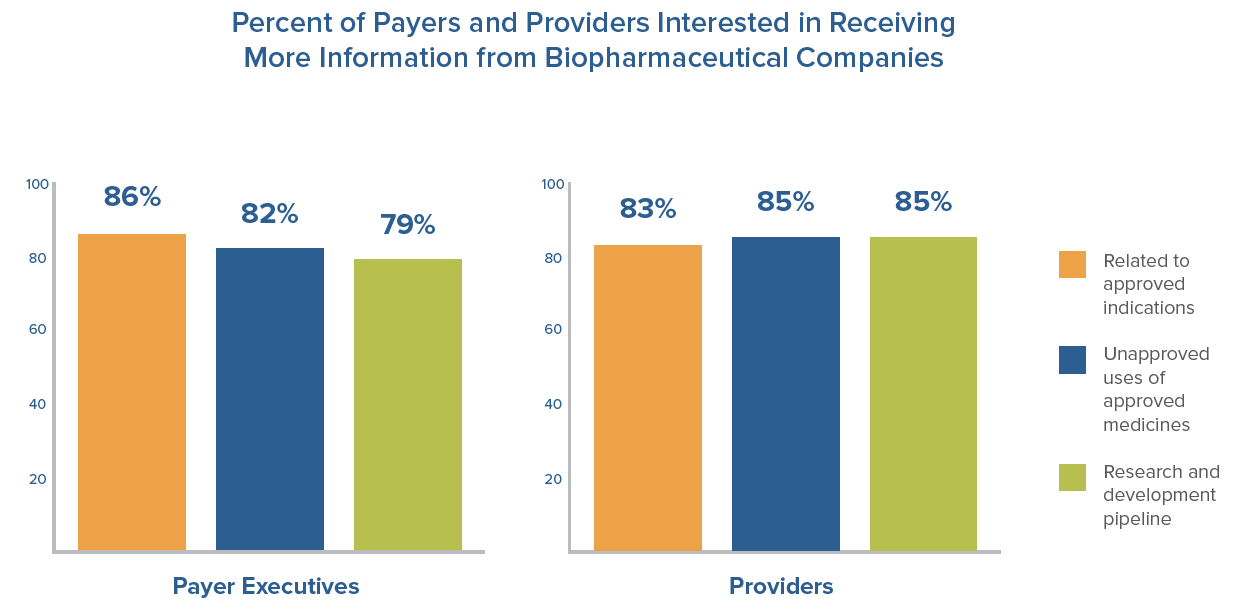
Despite the desire from payers and providers for increased data, FDA regulations can prevent biopharmaceutical companies from sharing important data about their medicines. FDA has begun to acknowledge this and has started taking steps to facilitate communications between biopharmaceutical companies and other key health care stakeholders. However, additional legislative or administrative changes are needed to ensure manufacturers are able to provide payers and providers with the most current and best available scientific information to inform their decision-making. With the right safeguards in place, allowing more flexibility for biopharmaceutical company communications with payers and providers can support better informed prescribing and more effective use of medicines, improving results for patients and protecting public health.
View the survey findings here.




