Rapid advances in scientific discovery have ushered in a new era of medicine, transforming our ability to treat, and in some cases cure, many of the most challenging diseases, including cancer, rare diseases and autoimmune conditions.
Over the past year, America’s biopharmaceutical companies have played a critical role in the global response to COVID-19. The unprecedented speed of developing new treatments and vaccines is due in large part to the productivity of the United States’ biomedical research ecosystem, which is sustained by a policy framework designed to support and advance the innovation of new medicines.
Let’s take a closer look at three key pillars of the biopharmaceutical ecosystem that are central to the success of America’s research and development (R&D):
1. Private-public collaboration
Before medicines can be available to patients, they begin as ideas that are based on investigation of newly discovered molecules, strange phenomena or little-understood processes in the body, commonly referred to as basic science research. This research, conducted by both the public and private sectors, is the foundation of our understanding of how the human body functions. However, ideas based on this basic science are still a long way from becoming new medicines.
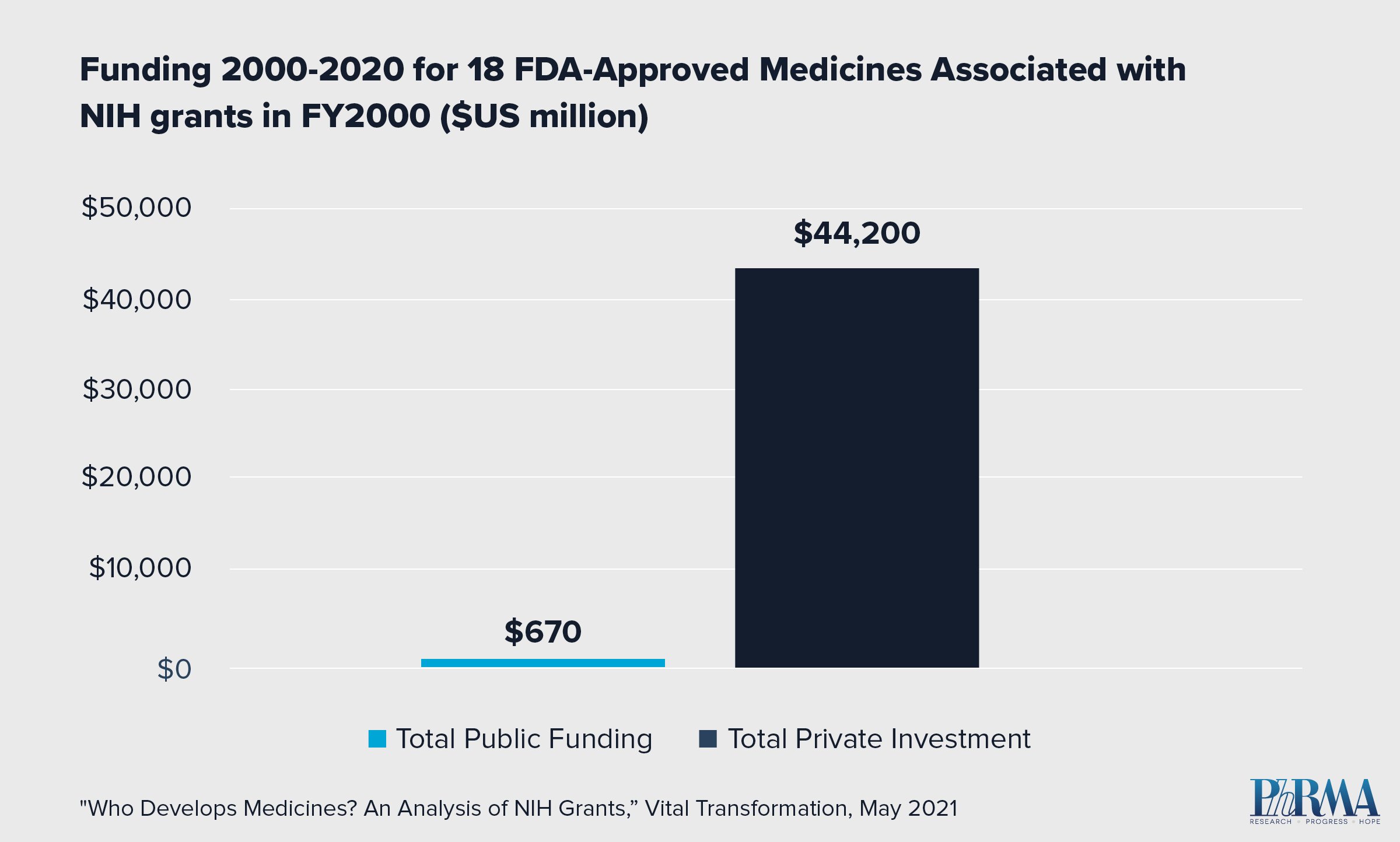
The federal government alone cannot generate new medicines without the resources, scientific expertise, R&D, manufacturing and technological platforms that private sector biopharmaceutical companies provide. In fact, a new report found that in a review of 23,230 National Institutes of Health (NIH) grants issued in the year 2000, there were grants linked to patents associated with only 18 U.S. Food and Drug Administration (FDA) approved medicines by 2020. Of those 18 medicines, none reached FDA approval without significant private sector investment to further the drug development process. Meaning in cases where NIH research is linked to patented discoveries, ongoing development of new, lifesaving and life-improving medicines would not be possible without the robust investment and shouldering of investment risk, scientific expertise and drug development and manufacturing capabilities of biopharmaceutical research companies. In fact, total private sector investment for the 18 approved medicines exceeded NIH funding by orders of magnitude: $44.2 billion in private investment compared to $670 million in NIH funding.
2. A robust intellectual property (IP) system
The U.S. IP system provides a framework for collaboration across all participants in the R&D ecosystem. One fundamental aspect of the U.S.’s strong IP system is the Bayh-Dole Act, passed by Congress in 1980, which incentivizes the private sector to make the substantial and risky investments needed to transform discoveries from government-funded basic scientific research into useful products. Specifically, it allows grant recipients, such as universities, to license patents to private sector partners while retaining the title to the patents. This relationship has had a strong impact on American R&D advancements. In fact, prior to enactment of the Bayh-Dole Act, the government retained the patents on federally-sponsored inventions – and only 5% of those patents were ever used in the private sector.
3. Strong private sector investment in R&D
The ongoing development of new, life-saving and life-improving medicines, including those for COVID-19, would not be possible without the robust investment from biopharmaceutical research companies. On average, it takes a decade or more and costs $2.6 billion to develop one new medicine, including the cost of many failures – less than 12% of new molecular entities that enter phase one clinical trials eventually receive FDA approval. According to a recent Congressional Budget Office report, the biopharmaceutical industry reported investments totaling $83 billion on R&D in 2019 alone. The same report found PhRMA members’ R&D spending since 2008 constitutes about 75-85% of the industry total. Overall, the biopharmaceutical industry’s investment in R&D and expertise resulting in a 60% increase in medicines that eventually gained approval between 2010 and 2019, compared with the previous decade.
With such high risks during drug development, a competitive marketplace that is underpinned by a strong policy framework supporting innovation is needed to ensure robust continued investment.
A hallmark of the U.S. R&D framework is the FDA human drug review program, which has set the global gold standard for regulatory review and approval. For nearly 30 years, the Prescription Drug User Fee Act (PDUFA) has played a critical role in strengthening the FDA’s ability to help ensure the availability of safe and effective medicines and meet urgent patient needs.
Now, more than ever, the biopharmaceutical ecosystem is vital to researching, developing and manufacturing life-saving medicines and treatments, and it is critical that policies support patient access and affordability without undermining the incentives and innovations to develop tomorrow’s lifesaving medicines. Through thoughtful, market-based approaches, we can continue to support a thriving biomedical research ecosystem and allow the biopharmaceutical sector to continue to deliver innovative medicines and improve the lives of patients in unprecedented ways.
For more information visit www.phrma.org/advocacy/research-development.