Before 2020, the importance of robust and resilient supply chains and how they can affect access to life-saving treatments probably wasn’t top of mind for many outside the biopharmaceutical industry. Then, in a sudden and very real way, the COVID-19 pandemic showed just how critical it is to have manufacturing facilities capable of scaling up quickly to meet an urgent public health demand. America’s biopharmaceutical companies rose to the challenge, researching and developing vaccines and therapeutics in record time, ramping up manufacturing to meet global demand and avoiding sustained supply chain disruptions.
Years of investment in the United States means more new medicines for patients are developed and made right here at home.
Here’s what you need to know:
- In recent years, the number of manufacturing facilities in the U.S. has grown by more than 50%. These facilities have been registered with the U.S. Food and Drug Administration to produce human-use medicines and the testing, labeling and packaging that goes with them. Today, biopharmaceutical companies continue to build, expand and upgrade facilities across the country to supply the next generation of cutting-edge therapies to American patients.
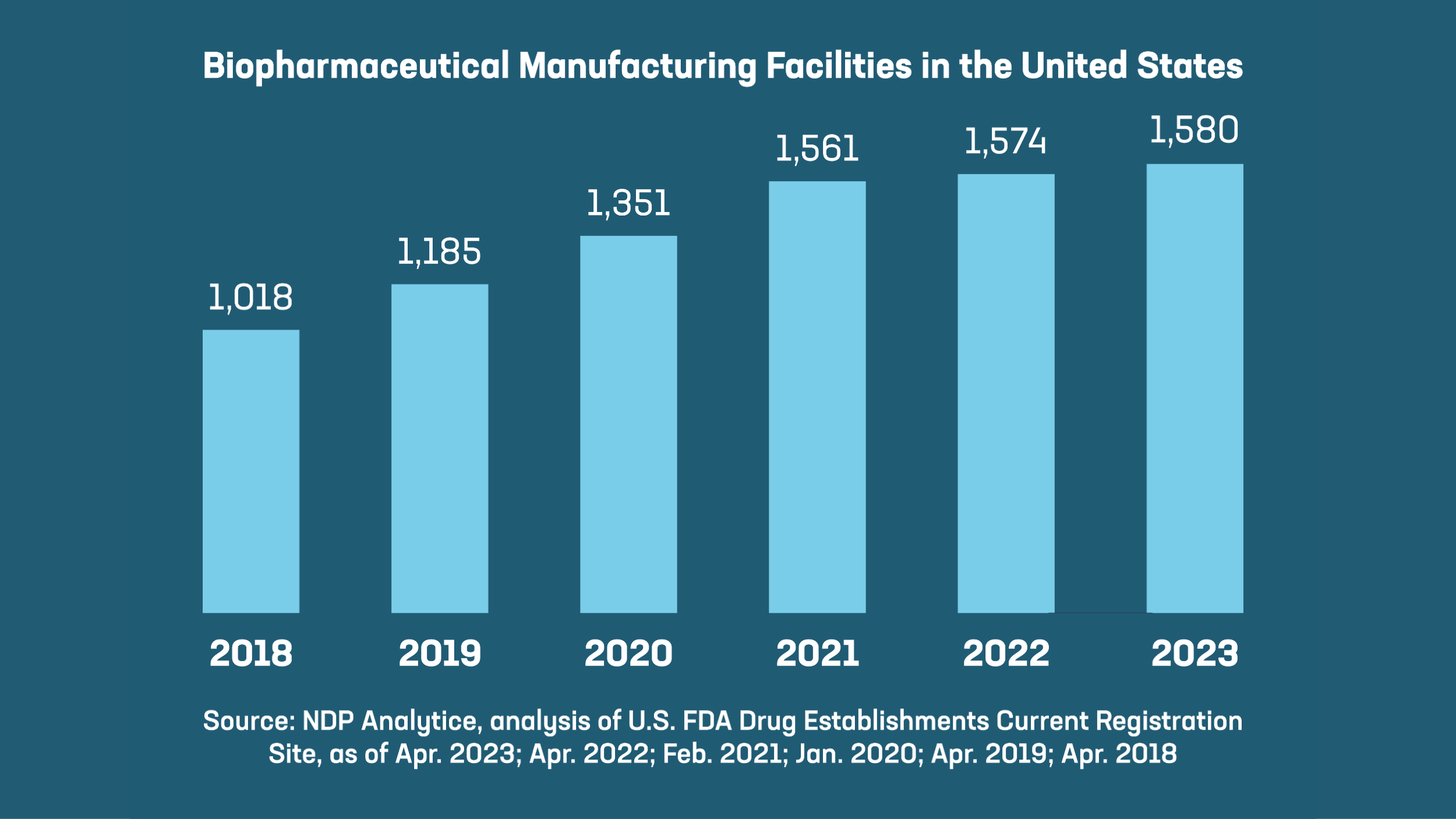
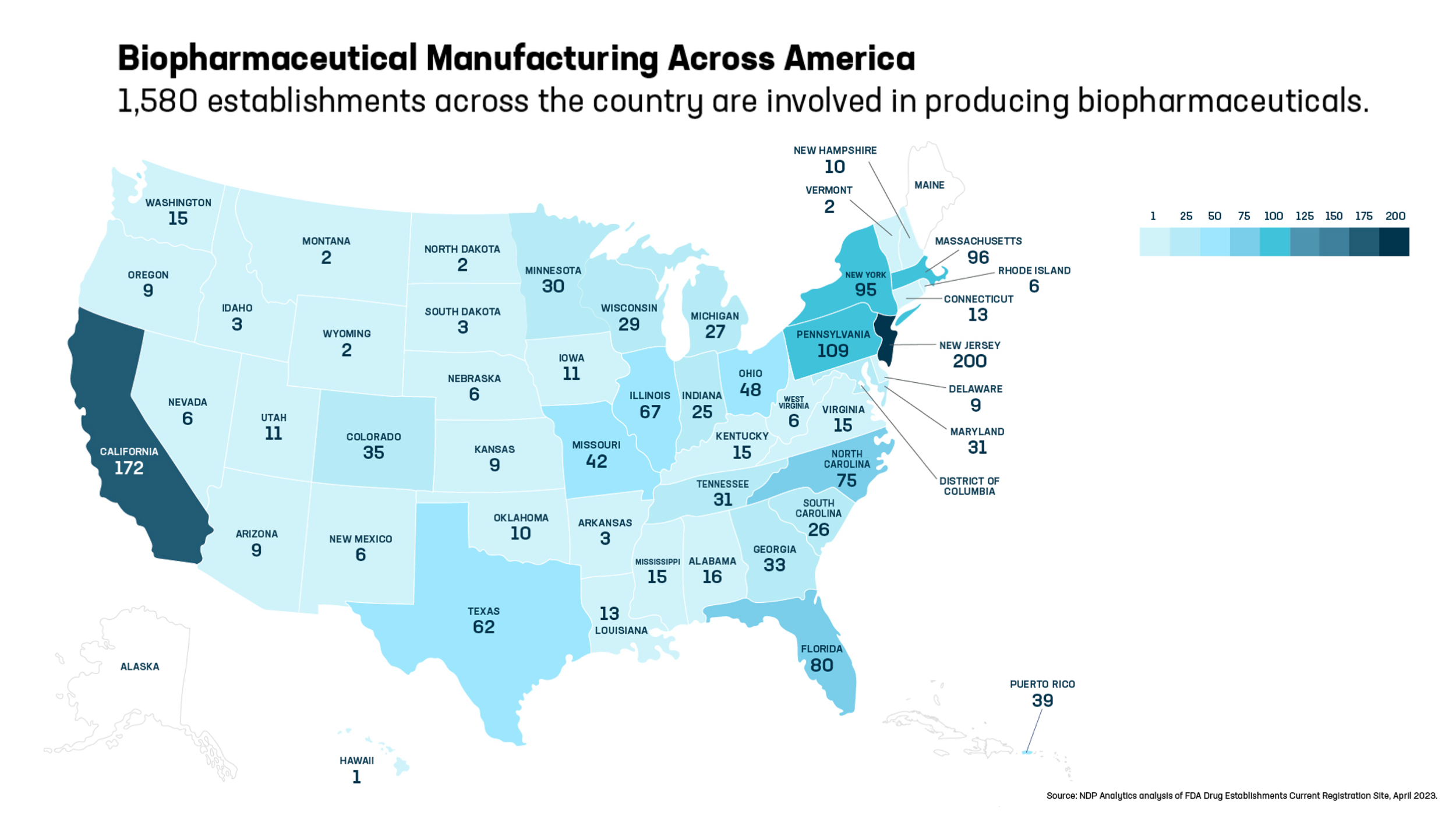
- As of April 2023, biopharmaceutical companies and their suppliers operated 1,580 production facilities across the country, in 48 states and Puerto Rico. Building just one facility can cost up to $2 billion and take five to 10 years before it is even operational, so this geographic reach and continued growth helps fortify the supply chains we all depend on and mitigate potential shortages or disruptions.
- Nearly 20% of these new facilities are manufacturing active pharmaceutical ingredients (APIs), which are the ingredients that produce a medicine’s intended health effects. While not the only part of the manufacturing process necessary to produce a medicine that can be delivered to patients, the production of APIs is an essential step in the process.
Investment in manufacturing in the U.S. helps to drive local economies, create jobs and boost American competitiveness. As companies add new manufacturing lines and expand existing lines to increase production capacity, their investments help ensure we continue to have resilient and secure supply chains to meet U.S. patient needs.
Click here to learn more about the role of manufacturing of biopharmaceuticals.





