The COVID-19 pandemic has left no person unaffected, but Black Americans have been disproportionately impacted. Black adults are nearly four times more likely to be hospitalized from the virus and nearly three times more likely to die from the virus than their white counterparts. At the same time, recent polls highlight COVID-19 vaccine hesitancy within Black communities. In fact, only one in four Black Americans (24%) plan to get the vaccine compared to other racial and ethnic groups, and Black Americans are significantly trailing in vaccination rates.
Contributing to vaccine skepticism are the significant systemic health disparities that Black Americans have faced for many years – disparities that the pandemic further exposed. Historic wrongs, such as the abuses of the U.S. Public Health Service Syphilis Study at Tuskegee and absence of consent in the Henrietta Lacks' story have seeded mistrust and had a lasting effect on the Black community.
Recent opinion research conducted by PhRMA[1] provides greater insight into some of the factors that contribute to mistrust and stand in the way of ensuring Black Americans have the information they need to make health decisions. One of the issues rising to the top of this research is a clear need for more diverse clinical trials, which is critical to accurately represent the intended patient population and achieving health equity. Currently, only one in 10 Black voters (13%) have participated in a clinical trial or know someone who has participated in a clinical trial for new medicines or treatments.
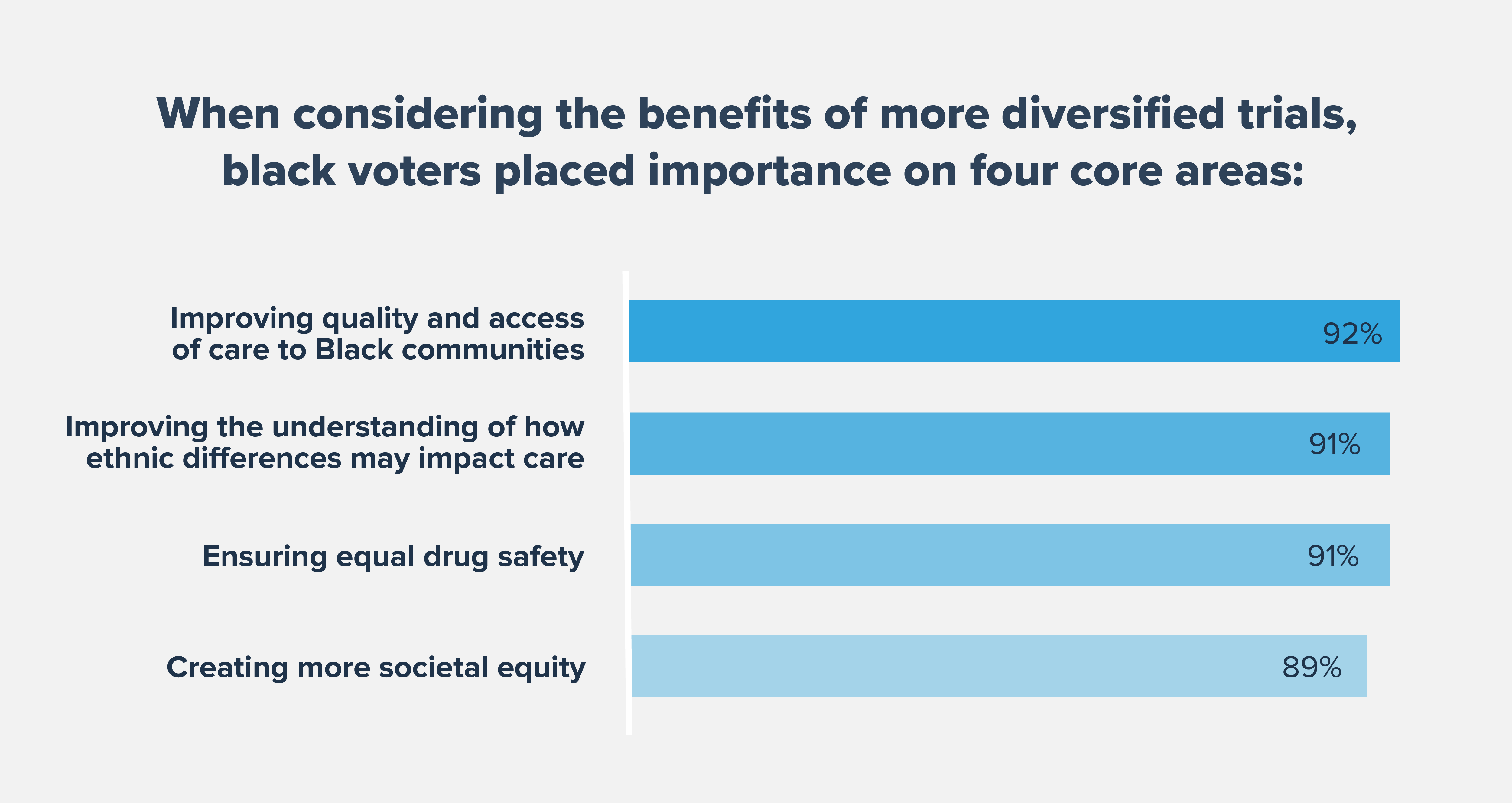
Improving representation in clinical trials is pivotal to combatting diseases that disproportionally affect Black Americans, such as diabetes, hypertension, heart disease and childhood asthma. When we polled Black voters, 89% say tackling these diseases with more diversified clinical trials is important and respondents named heart disease, diabetes, hypertension and cancer as diseases they would like to see prioritized for safe and diverse clinical trial research.
Encouragingly, Black Americans recognize that there are many short- and long-term benefits to greater diversity and representation in clinical trials. In a recent survey, significant majorities of Black voters say that safe trials can lead to improved health outcomes for Black Americans, including drug safety and an overall improvement in the quality of health care.
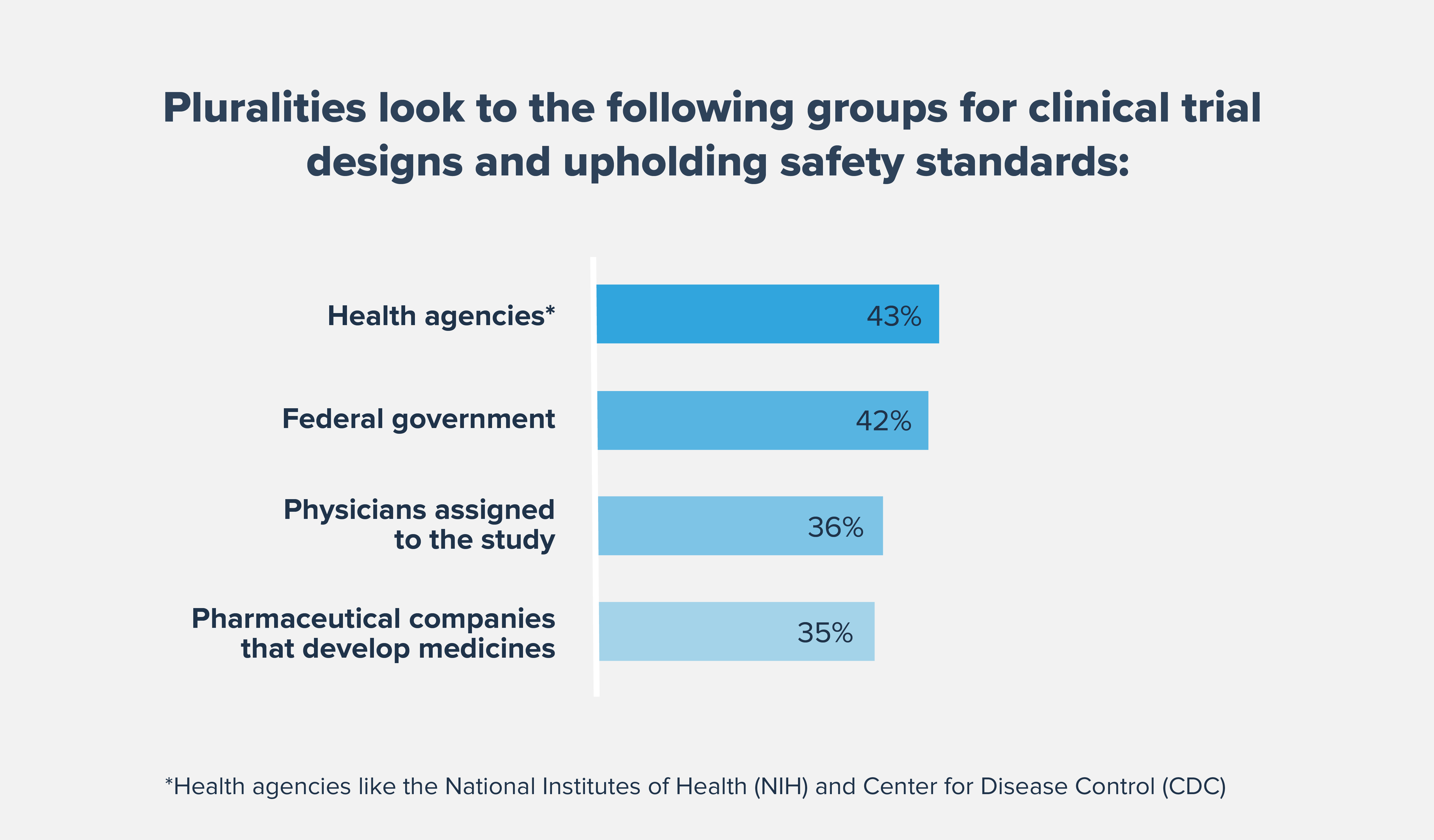
 We must continue to aggressively address underlying systemic biases and challenges created by historic wrongs and build community-wide trust to improve patient outcomes for underserved populations. Diverse clinical trials that accurately represent the intended patient population can help provide people with access to potentially lifesaving medicines and lead to evidence that better reflects the patients that are most likely to use the medicine if approved.
We must continue to aggressively address underlying systemic biases and challenges created by historic wrongs and build community-wide trust to improve patient outcomes for underserved populations. Diverse clinical trials that accurately represent the intended patient population can help provide people with access to potentially lifesaving medicines and lead to evidence that better reflects the patients that are most likely to use the medicine if approved.
Importantly, in 2020, PhRMA announced the first-ever, industry wide principles on clinical trial diversity. The principles focus on four main areas: building trust and acknowledging the historic mistrust of clinical trials within Black and Brown communities, reducing barriers to clinical trial access, using real-world data to enhance information on diverse populations beyond product approval and enhancing information about diversity and inclusion in clinical trial participation. These principles were approved by the PhRMA Board of Directors and take effect in April of 2021.
Systemic racism is as real as any disease. Diversifying clinical trials is a critical path to change and PhRMA, and its members, are dedicated to earning the trust and addressing the systemic issues that prevent Black communities from enrolling in clinical trials so that people who want to participate, can.
For more information, visit https://www.phrma.org/Equity.
[1] PhRMA Tracking Poll with Morning Consult, February 10-11 2021 (n=1984) registered voters (n=154 Black voters)
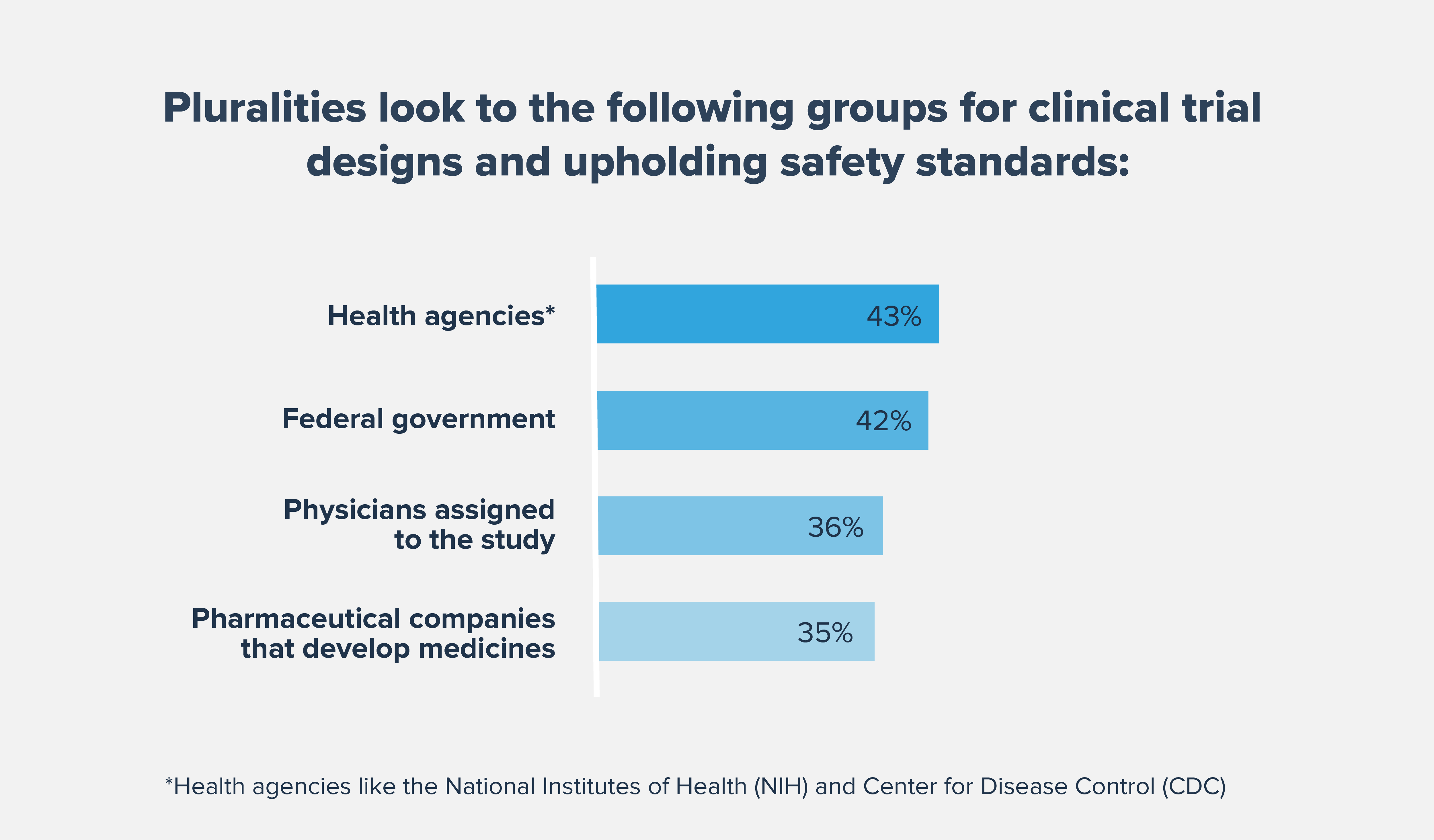



 We must continue to aggressively address underlying systemic biases and challenges created by historic wrongs and build community-wide trust to improve patient outcomes for underserved populations. Diverse clinical trials that accurately represent the intended patient population can help provide people with access to potentially lifesaving medicines and lead to evidence that better reflects the patients that are most likely to use the medicine if approved.
We must continue to aggressively address underlying systemic biases and challenges created by historic wrongs and build community-wide trust to improve patient outcomes for underserved populations. Diverse clinical trials that accurately represent the intended patient population can help provide people with access to potentially lifesaving medicines and lead to evidence that better reflects the patients that are most likely to use the medicine if approved.