The United States has led the charge in the development of vaccines and treatments to combat COVID-19 thanks in large part to our robust innovation ecosystem, supported by intellectual property (IP) protections. The decision made by the Biden Administration and other WTO members earlier this year to waive commitments to protect IP on COVID-19 vaccines under the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement was harmful and unnecessary and removed the very incentives that enable scientific advancement. Expanding the TRIPS waiver to include COVID-19 treatments — as some WTO members wish to do — would jeopardize American innovation and our ability to prepare for future pandemics.
Robust research and development is still happening to invent medicines to prevent and combat COVID-19’s impacts on the human body. Currently, 28 PhRMA member companies have more than 150 unique COVID-19 products being studied in more than 2,000 clinical trials: 451 investigating vaccines and 1,559 investigating treatments. Expansion of the TRIPS waiver would jeopardize the 97% of medicines in development for COVID-19 that have yet to be launched.
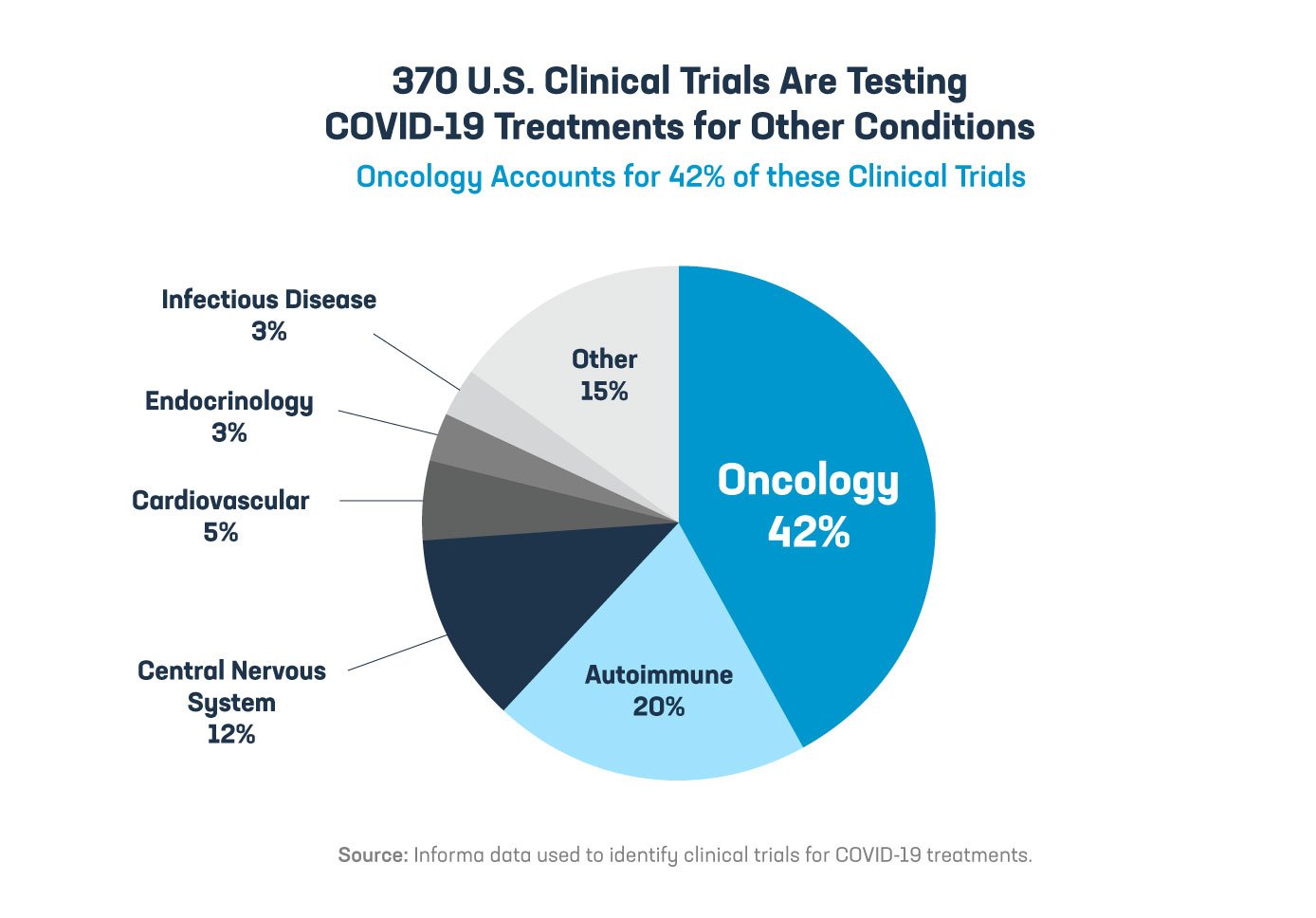
Hundreds of medicines being developed to protect patients from severe illness resulting from COVID-19 have potential beyond this virus. These same medicines are also being researched and developed to potentially treat cancer, HIV and neurological diseases such as Alzheimer’s, multiple sclerosis and Parkinson’s. If the TRIPS waiver was expanded to treatments, effectively giving away American innovation to foreign competitors, then it would undermine incentives to develop these potentially life-saving therapeutics for millions of patients around the world.
Beyond COVID-19, the ability to prepare for and respond to future pandemics and health crises relies on collaborations and innovations. The United States is the global leader of biopharmaceutical innovation because America’s strong IP system promotes competition and incentivizes risk taking. Most foreign governments already offer significantly weaker IP protections, including for American-made products. Weakening those protections further, by waiving commitments to protect IP for certain products at the WTO, sets an alarming precedent. Expanding the TRIPS waiver to include treatments will harm medical progress by discouraging American companies from researching and developing future treatments and cures.
Rather than hamper American innovation and leave the world more vulnerable to future pandemics and crises, the Biden Administration and governments everywhere should support a strong global IP ecosystem and focus on the real barriers standing in the way of COVID-19 medicine access, such as regulatory challenges, last-mile distribution hurdles and poorly-funded public health systems in many low- and middle-income countries.
To read more about the harms of TRIPS waiver expansion, click here.




