Over the past 25 years, prescription medicines have transformed the trajectory of many debilitating diseases and conditions, including heart disease, HIV/AIDS, cancer and hepatitis C, resulting in decreased death rates, longer life expectancy and better quality of life for patients.
Today, new medicines are targeting the underlying causes of disease in ways never seen before, and diseases previously regarded as deadly are now manageable and even curable. In this new era of medicine, breakthrough science and personalized therapies are revolutionizing the way we treat patients with a broad range of chronic and rare conditions. Looking forward, continued advances in biopharmaceutical innovation are critical in addressing patient’s unmet needs, improving public health and solving future health care challenges.
But the ecosystem that supports the development of new treatments and cures is complicated. Let’s take a look at a few facts about the development of innovative new medicines and how laws put in place are helping support continued innovation:
- The public and private sectors play complementary roles with government having a largely indirect role in medicine development. While government research often lays the groundwork for developments in medical science, including basic understanding of disease, so too does the biopharmaceutical industry. Biopharmaceutical companies support a range of basic research, not only in their own labs, but in partnership with academia and small and emerging companies. Moreover, biopharmaceutical companies are largely responsible for developing new medicines. One study found that the private sector was responsible for 58% of discovery milestones, 73% of development milestones and 81% of manufacturing milestones.
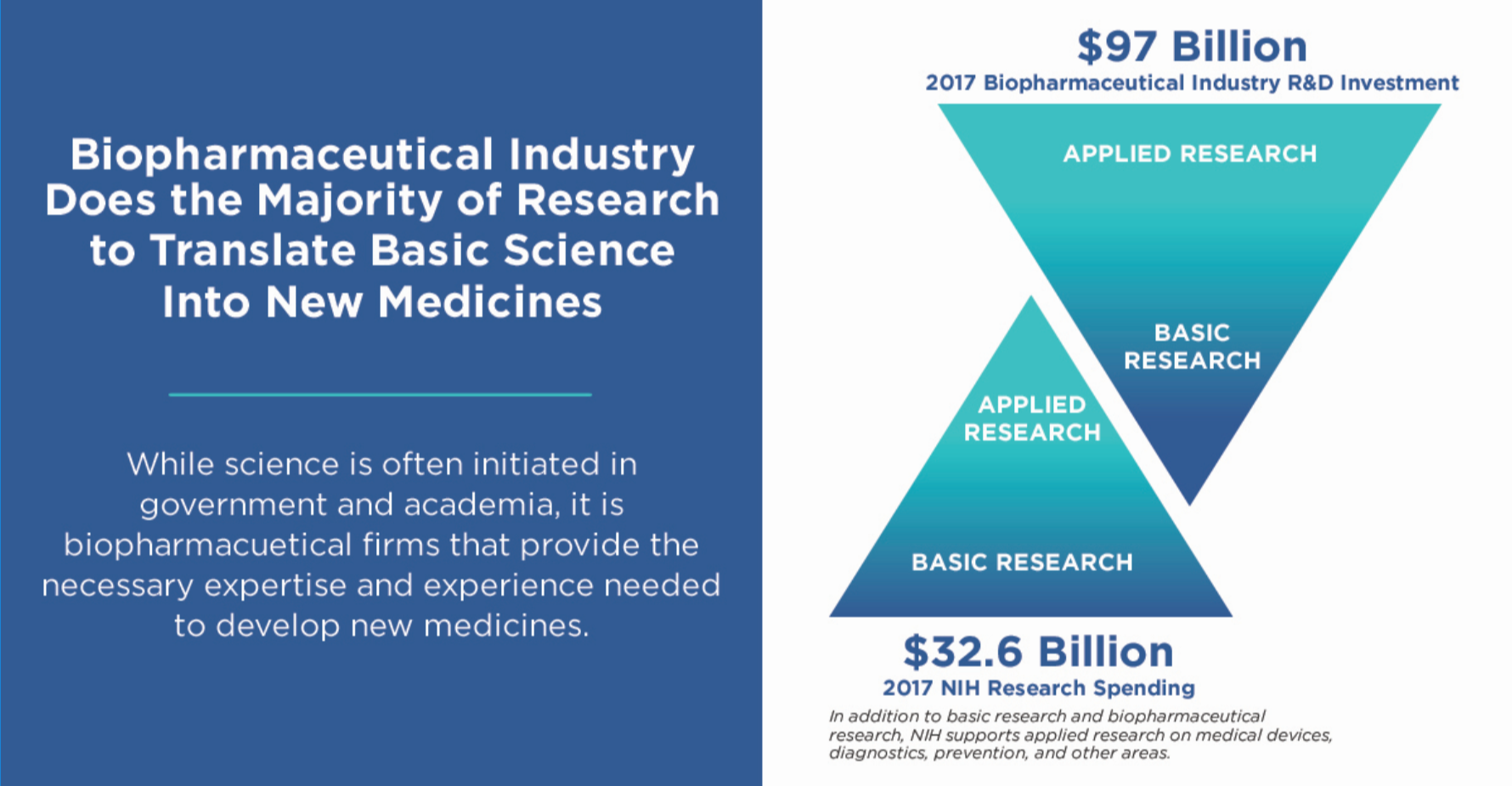
- Translating basic research into new medicines is a long, difficult and expensive process that is taken on by biopharmaceutical companies. The biopharmaceutical industry greatly outspends government and academia when it comes to investment in research and development (R&D). According to most the recent data, in 2017 alone, the biopharmaceutical industry’s investment in R&D reached an estimated $97 billion, which almost triples the National Institutes of Health (NIH) total budget of $32.6 billion in 2017, only part of which goes to research related to drug development.
- In cases where federally-funded research results in a patentable invention that has potential to develop into a therapeutic treatment, laws such as the Bayh-Dole Act have created a framework for translating government-funded research into useful commercial products. This includes allowing institutions that conduct federally-funded research to retain title to the patents covering inventions they have created. This enables these research institutions to license the patents to private sector partners, including biopharmaceutical companies, who then work to develop these inventions into promising treatments or cures. This can lead to royalties to the research institution once commercialization is achieved by biopharmaceutical companies.
- The impact of Bayh-Dole has been far reaching — bolstering the economy and positioning the U.S. as a global leader in technology transfer— that other countries now seek to emulate. Between 1996 and 2015 alone, the licensing activity spurred by Bayh-Dole contributed close to $591 billion to U.S. gross domestic product and supported an estimated 4.2 million jobs in the U.S. In 2016 alone, more than 1,000 start-up companies were formed and nearly 800 commercial products stemming from university research were introduced.
- Bayh-Dole has created an efficient route by which new insights and valuable research results from universities and other institutions can effectively make their way to start-up and established firms across different sectors who assume the full risk and cost of working to transform these inventions into technologically and economically viable products.
- Ultimately, Bayh-Dole has played a key role in not only supporting innovation. But as a Congressional Research Service report noted, “Observers generally agree that the Bayh-Dole Act has successfully met its objectives…The government receives a significant payback through taxes on profits and society benefits from new jobs created and expanded productivity.”
To ensure the continued promise of biopharmaceutical innovation, the current technology transfer framework must be protected. In the same way that the U.S. R&D ecosystem did not develop by accident, it cannot continue without committed support from all stakeholders. It will take intentional, collaborative efforts from policymakers, industry leaders, institutional researchers and patients to ensure the U.S.’ capacity for innovation not only thrives but continues to grow in size and scope.