New government price setting policy threatens post-approval research
Patients are the ones harmed by the Inflation Reduction Act’s price setting policy.

New government price setting policy threatens post-approval research.
Patients are the ones harmed by the Inflation Reduction Act’s price setting policy.

New government price setting policy threatens post-approval research.
Have you ever noticed that one medicine might be used to treat two, three, seven different conditions? That’s the result of significant additional research and investment, including lengthy and costly clinical trials, biopharmaceutical companies undertake after a medicine is initially approved by the Food and Drug Administration (FDA). Such post approval R&D has changed the course of treatment for many diseases and patient populations. Despite this, the recently enacted Inflation Reduction Act disincentivizes post-approval R&D, and as a new analysis demonstrates, this is very concerning for patients across the country.
The Partnership for Health Analytic Research looked at 88 medicines that received initial FDA approval between 2010 and 2012 and analyzed the post-approval indications those medicines received. Key findings include:
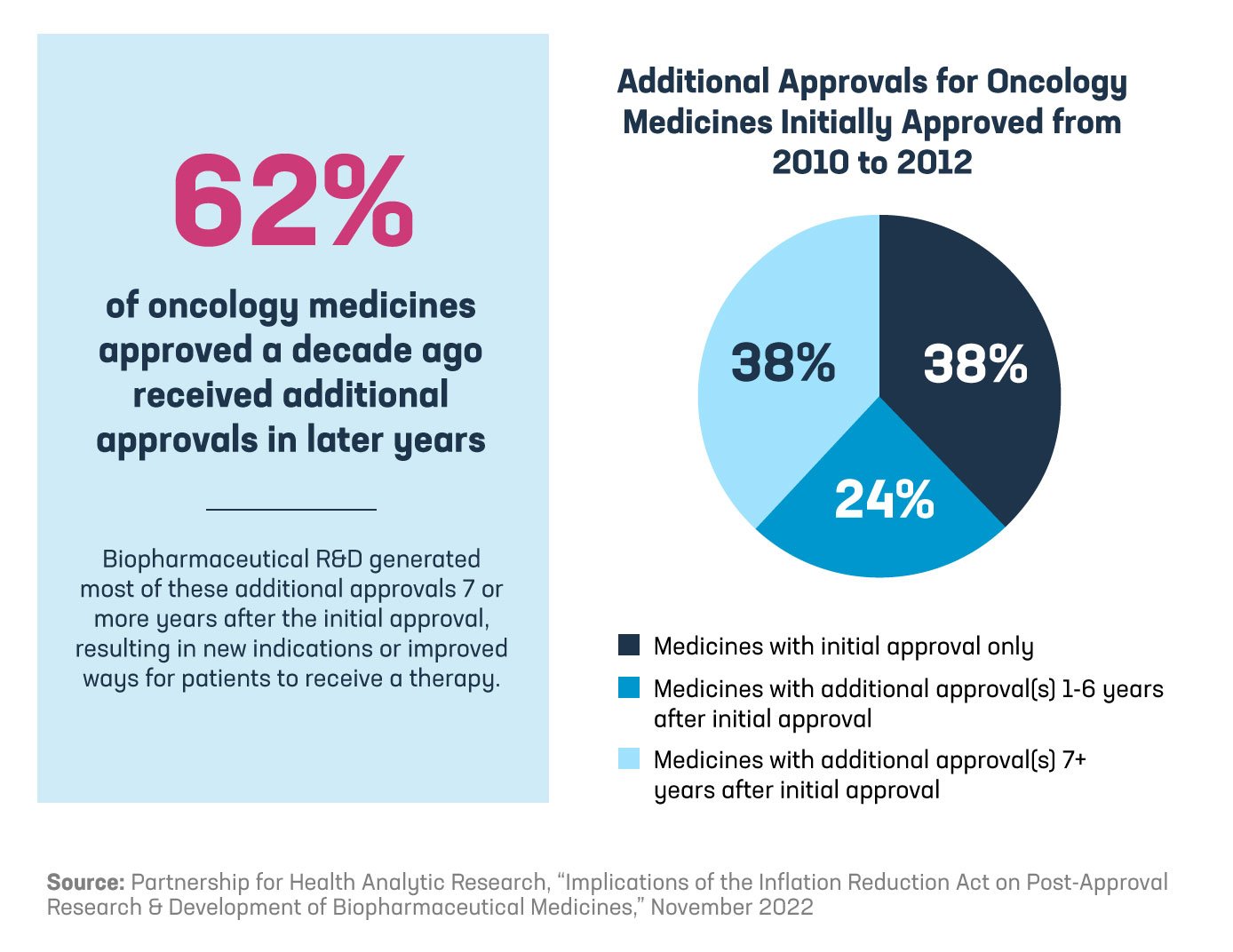
The Inflation Reduction Act, however, relies on pre-defined times after a medicine is initially approved to impose a government set price, ignoring the natural R&D process that continues in the years following a medicine’s approval. It also ignores the timeline for IP protections put in place by Hatch-Waxman and the Biologics Price Competition and Innovation Act that help strike the balance between incentivizing innovation and facilitating additional competition in the marketplace. Specifically, small molecule medicines could be selected by the government for price setting as early as 7 years after initial approval, with the price being set as early as 9 years after approval. And biologics could be selected by the government for price setting as early as 11 years after initial approval, with the price being set as early as 13 years after approval.
As the PHAR analysis demonstrates, many medicines received additional indications 7 years or more after approval. What incentive do biopharmaceutical companies have to invest in post-approval research if the government can set the price long before they even complete the research and can obtain approval for the additional indications?
In the end, patients are the ones harmed by the Inflation Reduction Act’s price setting policy. There are so many patients and diseases with unmet medical need, yet we now have a U.S. law in place that interferes in ongoing R&D efforts to find new treatments and cures for patients. Policymakers should take a step back and consider if government price setting policies are in the best interest of Americans.
Read the full PHAR analysis here and learn more about the negative impacts of the Inflation Reduction Act here.