New report finds 15 new oncology therapies launched last year
The report also highlights the dozen immunotherapy indications approved since the first immunotherapy was approved in 2014

New report finds 15 new oncology therapies launched last year.
The report also highlights the dozen immunotherapy indications approved since the first immunotherapy was approved in 2014

New report finds 15 new oncology therapies launched last year.
A record 15 new oncology therapeutics launched in 2018 for 17 tumor types, according to IQVIA’s “Global Oncology Trends 2019” report. This progress includes a continued shift toward precision medicine, where therapeutics target the genetic profile of cancer patients rather than site-of-origin in the body.
The report also highlights the dozen immunotherapy indications approved since the first immunotherapy was approved in 2014. These therapies, which use a patient’s own immune system to identify and target cancer cells, “represent some of the most advanced types of treatments available to patients with cancer,” according to the report.
Despite these leaps in cancer treatment, spending on these therapies continues to represent a small and stable share of overall health care spending. The report found that spending on cancer medicines accounted for less than 2% of total national health expenditures in 2017. This remains true even with increased cancer prevalence and survival rates.
The report also found tremendous growth in the oncology pipeline, particularly among immunotherapies and precision medicines. The report found:
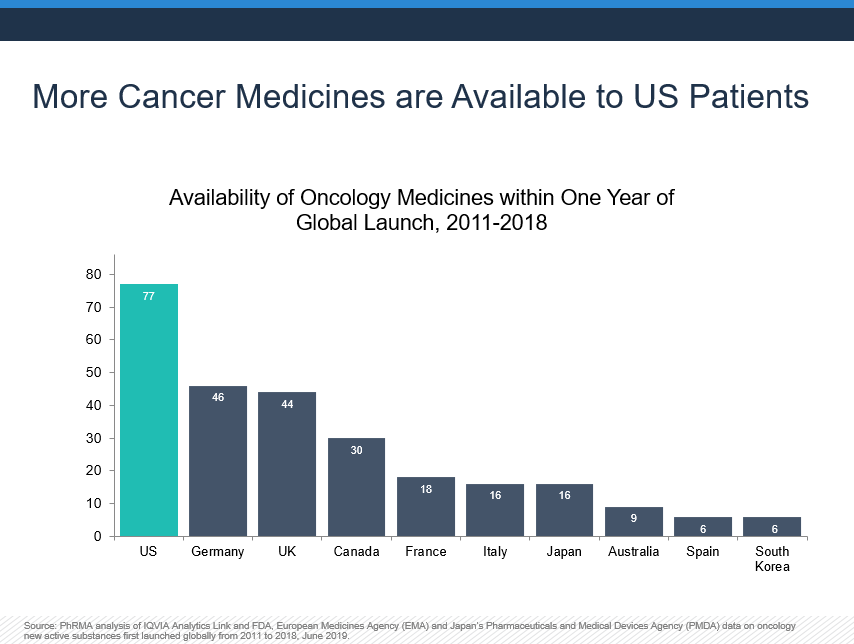
In this new era of medicine, patients in the United States have access to more new treatments, gain that access sooner and have better outcomes—including in cancer survival—than in other countries where governments restrict patient access. The report found access to recently launched oncology medicines remains low outside the United States. These findings are supported by another analysis of IQVIA data that found, among the 86 new oncology medicines launched globally between 2011 and 2018, 90% were available in the United States within a year of global first launch compared to just 53% for Germany and even fewer in other countries.
In light of these tremendous advances in cancer treatment and the amount that stands to be gained from the potential new medicines in the oncology pipeline, it is critical that policies in the United States support the next generation of treatments and cures. Learn more here.