Chronic diseases like Alzheimer’s disease, diabetes and kidney disease disproportionately affect older adults. In the United States, nearly 95% of older adults have at least one chronic condition and almost 80% have two or more. Chronic diseases can often limit an older adult’s ability to work, perform daily activities, cause them to lose their independence and lead to the need for long-term care. Furthermore, the economic burden imposed by chronic conditions is substantial, but this burden is concentrated among older Americans covered under Medicare. In fact, the combined costs of managing chronic diseases account for two-thirds of all health care costs and 93% of Medicare spending.
In light of the growing burden chronic diseases have on older adults and our broader health care system, America’s biopharmaceutical industry has made notable progress over the past several decades in researching and developing new medicines for chronic diseases, including those disproportionately affecting older adults. But considerable unmet medical need remains as many older adults continue to lack treatment options or therapies that would better meet the wide range of complex needs that these patients face.
Today, PhRMA released a new report covering more than 400 medicines in development that are targeting common chronic diseases impacting older adults, all of which are in clinical trials or awaiting review by the U.S. Food and Drug Administration (FDA). These medicines in the pipeline hold the potential to further address unmet medical needs, including:
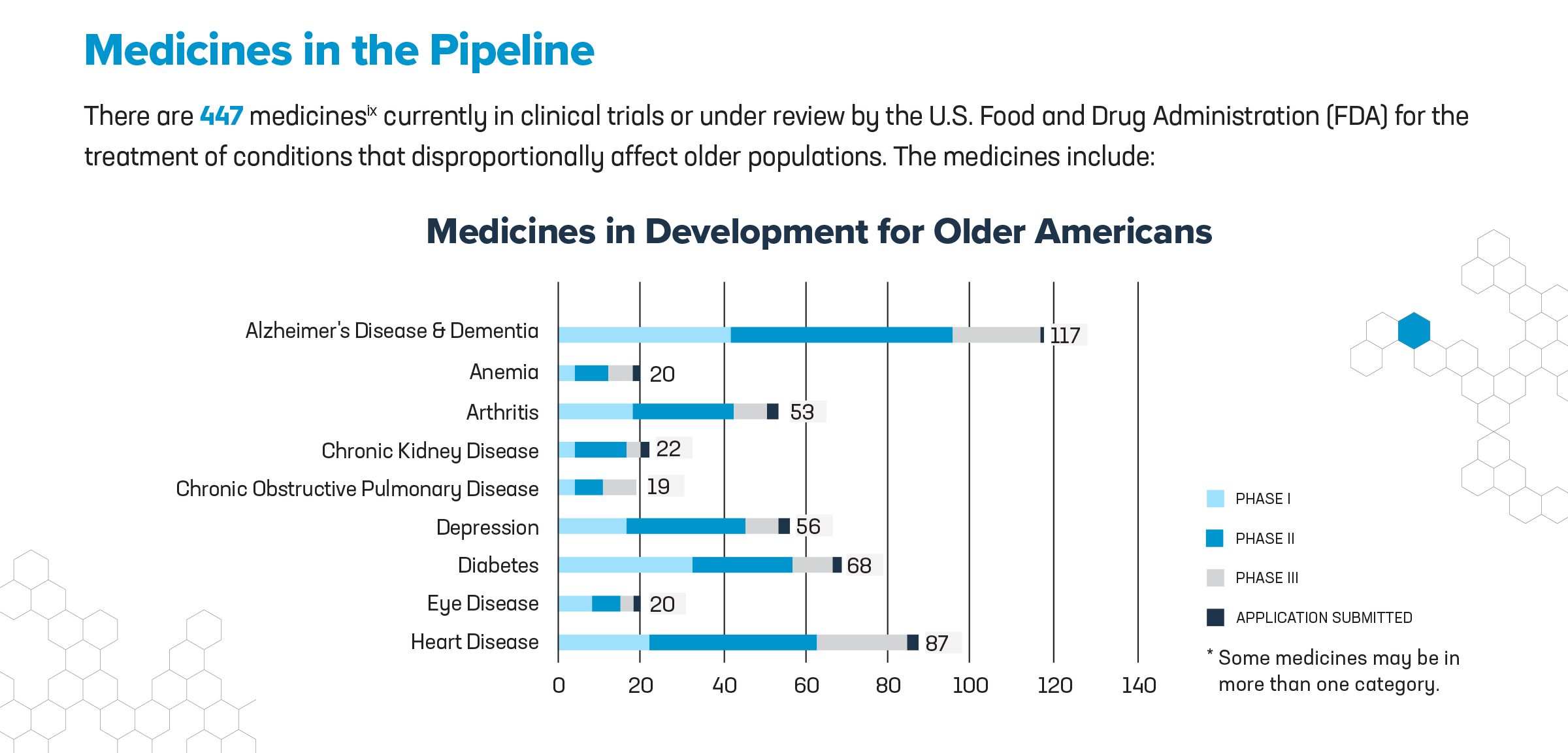
The medicines in development today underscore the biopharmaceutical industry’s commitment over the past several decades to research and develop (R&D) new medicines for older adults with chronic diseases. While the pipeline for chronic diseases is promising, recent price setting policies could affect R&D of new medicines and potentially deter efforts to meet the unmet needs of older adults.
The Inflation Reduction Act (IRA) passed in late 2022 included price setting provisions allowing the government to set the price of medicines covered by Medicare. The provisions ignore the natural R&D process and lay out a predetermined timeline after a medicine is initially approved, at which point the government can select a medicine for price setting. This discourages R&D for medicines impacting the Medicare population because it forces companies to decide whether it is worth investing time and resources into their development and whether they can recoup their costs. This also ignores the additional R&D that continues in the years following initial approval, the type of R&D which often leads to critical advances in treatment for patients with chronic diseases.
In a survey, PhRMA member companies expressed concern with how price setting undermines current incentives for many advances critical to patients, including our nation’s seniors. When asked, 82% or more of companies with pipeline projects in cardiovascular, mental health, neurology and cancers expect “substantial impacts” on R&D decisions in these areas.
While the IRA will have a chilling effect on biopharmaceutical efforts to meet the unmet needs of patients, biopharmaceutical companies will continue to explore cutting-edge research in the pursuit of new medicines but will have to make tough choices on which therapeutic areas to invest in. To continue to advance medical discovery for older adults, policymakers should make it a priority to preserve innovation in life-saving treatments and medicines for chronic diseases and the innovation that older Americans depend upon. That means an environment that is supported by a science-based regulatory system, strong intellectual property rights and a recognition that price setting policies jeopardize both the engine of American innovation and a robust pipeline of novel medicines to address unmet needs.
View the full report and more here.




