PDUFA 101
PDUFA has helped provide patients with more timely access to innovative, safe and effective medicines to combat diseases.

PDUFA 101.
PDUFA has helped provide patients with more timely access to innovative, safe and effective medicines to combat diseases.

PDUFA 101.

With Prescription Drug User Fee Act (PDUFA) VI FDA-Industry negotiations about to commence, we’re taking a look at what PDUFA is, how it works, and explaining why it has been a tremendous success for patients. PhRMA and the Biotechnology Industry Organization (BIO) will partner in negotiations, alongside consumer advocacy groups, health care professionals and scientific and academic experts. The U.S. Food and Drug Administration (FDA) initiated the PDUFA reauthorization process through a public meeting on July 15, 2015.
For more than 20 years, since enactment by Congress in 1992, PDUFA has helped provide patients with more timely access to innovative, safe and effective medicines to combat diseases, such as cancer, cardiovascular disease, infectious diseases and more. PDUFA provides FDA with necessary resources to meet performance goals for the regulatory review of new medicines. Here are some key dates:
PDUFA has played a critical role in bolstering the FDA’s ability to regulate safe and effective medicines for patients. PDUFA was created in response to a perilous bottleneck of new drug approvals in the late 1980s and early 1990s that left patients waiting, and sometimes dying, while an understaffed and underfunded FDA struggled to review new drug applications. In 1992, Congress passed the first PDUFA to meet urgent patient demands for more timely approvals of life-saving medicines.
For more than 20 years, PDUFA has helped the FDA fulfill its central mission – to protect and promote the public health by allowing the Agency to keep pace with the rapid increase in the number and complexity of innovative drugs and biologics entering the drug development and regulatory review pipeline.
The PDUFA program has enabled FDA to hire additional staff to review applications for new drugs and biologics. In 1989, FDA’s Human Drug Review Program was staffed by approximately 1,900 employees – by 2014, the human drug review staff had grown to more than 3,700.
The history
The origins of PDUFA stem from the emerging AIDS epidemic in the 1980s which sparked demand for faster review times on new treatments and therapies. Patient activists argued that because there were few treatments for AIDS, new drugs should be reviewed as quickly as possible. Those protests helped push FDA, Congress and the biopharmaceutical industry to work together to shorten review times. As a result of PDUFA and the New Molecular Entity (NME) Review Model which was implemented in 2012, average review times for innovative medicines have decreased significantly to 6.5 months for a priority review and 12 months for standard review NME application.
How it works
Biopharmaceutical companies pay three different user fees under PDUFA:
Reauthorization timeline
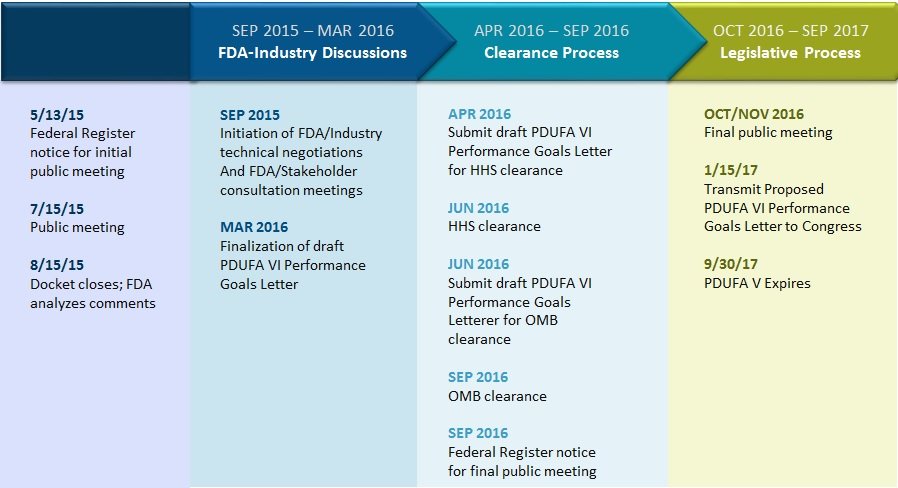
Reauthorization of PDUFA is critical to ensuring America’s biopharmaceutical companies can continue scientific innovation and bring new treatments to help patients live longer, healthier lives. To learn more visit the FDA’s PDUFA resource page. To learn more about the drug development process check out Innovation.org.