Policies to combat prescription drug abuse and misuse
Each day, almost 7,000 people are treated in emergency departments for using prescription opioid drugs in a manner other than prescribed.
Policies to combat prescription drug abuse and misuse.
Each day, almost 7,000 people are treated in emergency departments for using prescription opioid drugs in a manner other than prescribed.

Policies to combat prescription drug abuse and misuse.
There has been a lot of discussion recently about prescription drug abuse, and rightfully so. Each day, almost 7,000 people are treated in emergency departments for using prescription opioid drugs in a manner other than prescribed.
Prescription medicines can and do improve and save lives when they are used appropriately. But when they are diverted, abused or misused, devastating consequences can result. There is perhaps no other health care challenge as complex and multifaceted and we all must work together to address this issue.
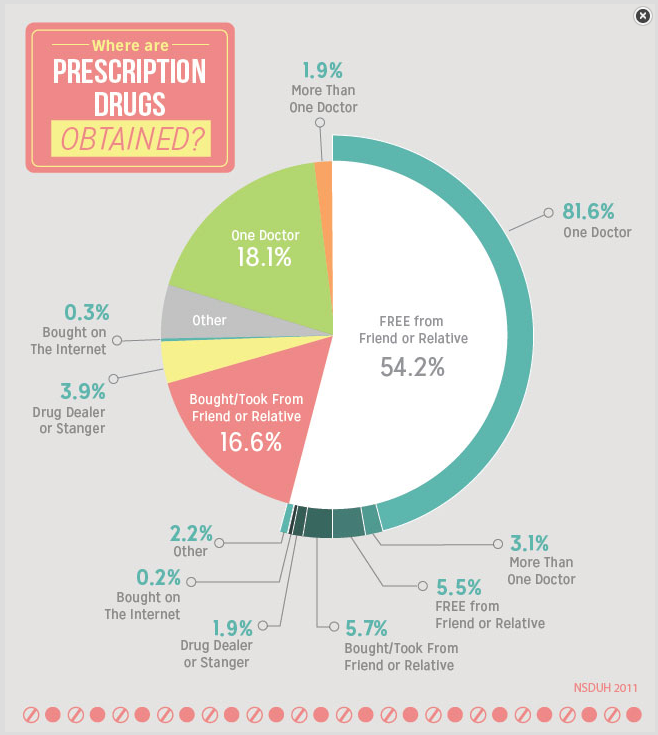
Infographic source: DrugAbuse.gov Link to page
As part of these efforts, the PhRMA and the innovative biopharmaceutical industry advocate for policies that will help combat prescription drug abuse and misuse, while also ensuring access to these medicines for patients with legitimate medical needs:
Ending the abuse and misuse of medicines is important to all of us, and we will continue to advocate for policies that will address this threat to public health. Learn more about how PhRMA and its member companies are working to prevent the misuse and abuse of prescription medicines.