Record number of personalized medicines approved in 2017
Advances in personalized medicine are making it possible to treat illnesses more effectively and efficiently.

Record number of personalized medicines approved in 2017.
Advances in personalized medicine are making it possible to treat illnesses more effectively and efficiently.

Record number of personalized medicines approved in 2017.
We are in an exciting time in biomedical innovation, as rapid scientific and technological advances are leading to new ways of preventing, diagnosing, and treating disease. Nothing illustrates the trajectory of the science better than the incredible growth we have seen in the field of personalized medicine.
Personalized Medicine at FDA: 2017 Progress Report, a new analysis from the Personalized Medicine Coalition, shows that 2017 was a record year for personalized medicine approvals. The U.S. Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) approved 16 personalized medicines in 2017, representing 34 percent of the 46 new medicines approved by CDER last year. These advances include:
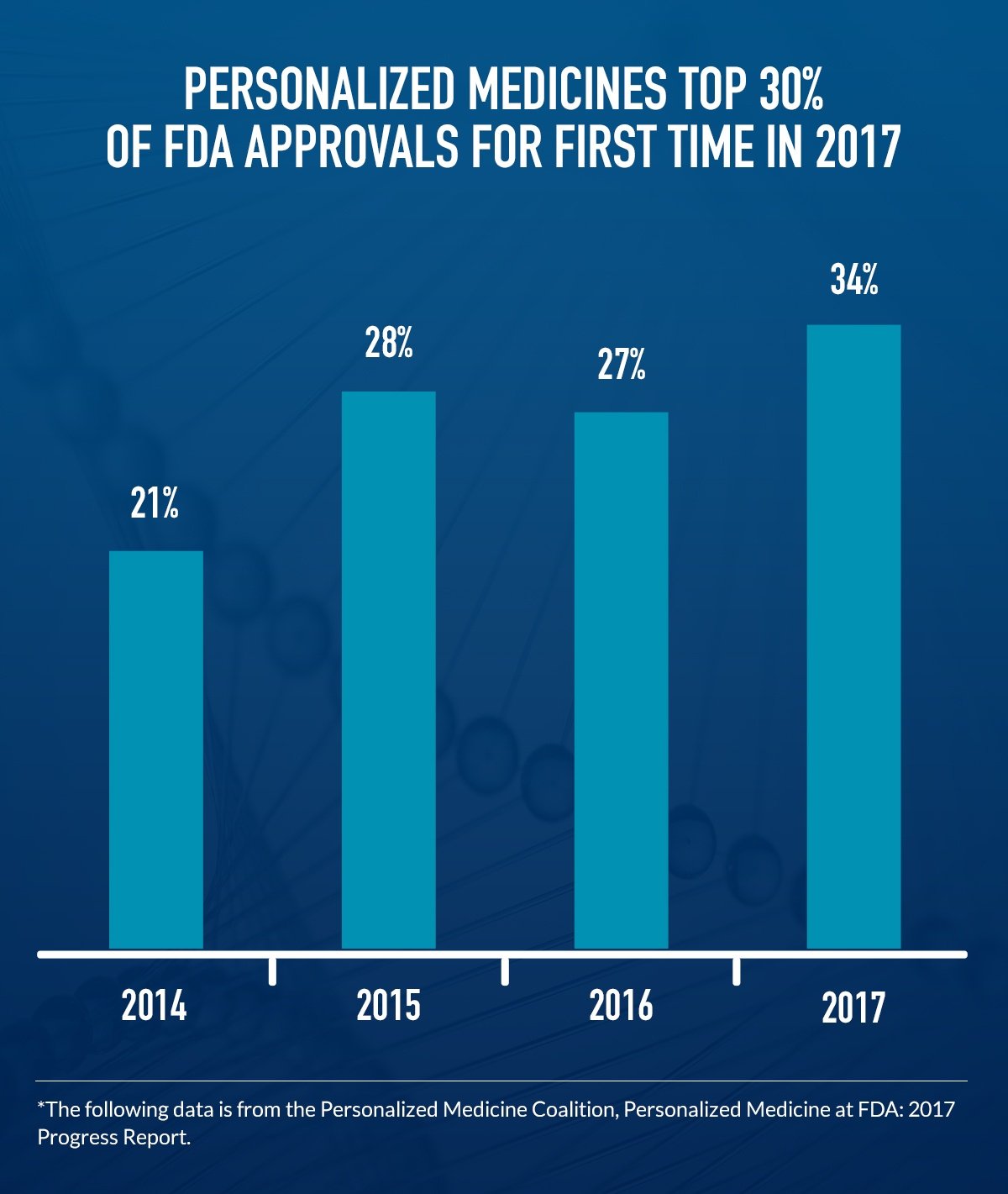
In addition to the landmark number of personalized medicines approved at CDER, the FDA’s Center for Biologics Evaluation and Research approved the first ever gene therapies in the U.S. in 2017, opening the door to a new paradigm of truly individualized treatments. These three treatments offer new, much needed options for patients with acute lymphoblastic leukemia, large B-cell lymphoma and retinal dystrophy (which causes blindness in children).
Just a decade ago, less than 10 percent of annual CDER approvals were personalized medicines. From only a handful of medicines to fully one-third of CDER approvals in 2017, the new data reflect not only the rapid pace of the science, but also researchers’ ongoing commitment to harnessing these advances in discovering and developing new medicines.
These approvals were part of an unprecedented wave of therapeutic advances in 2017, with both new medicines and expanded uses of already approved medicines opening important doors for patients.
Looking ahead, more than 42 percent of medicines in development by biopharmaceutical researchers have the potential to be personalized medicines. In cancer, a remarkable 73 percent may be personalized medicines, reflecting the major impact genetic and molecular science is having on oncology research.
These advances in personalized medicine are making it possible to treat illnesses more effectively and more efficiently, showing tremendous promise for the future of medicine.