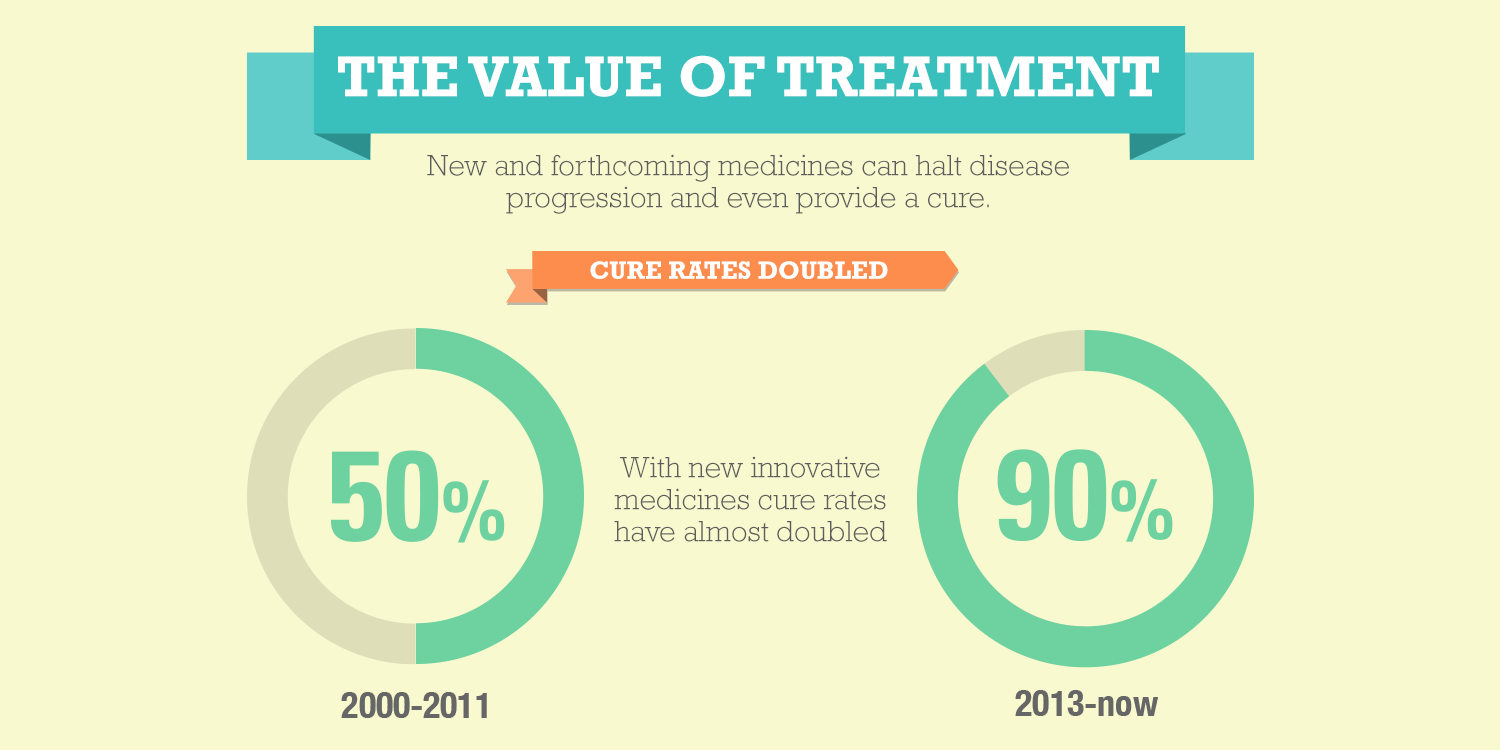
Today is Rare Disease Day, an annual reminder of the incredible community of patients living with a rare disease. In the U.S., rare diseases are classified as conditions that affect fewer than 200,000 people, and according to the National Institutes of Health (NIH), 10 percent of the U.S. population currently lives with a rare disease. To date, researchers have identified more than 7,000 rare diseases, 85 percent of which are considered life-threatening, and the biopharmaceutical industry is committed to finding treatments for these 30 million patients living with rare diseases. We’ve made important progress toward diagnosing and treating rare diseases, yet many unmet medical needs still remain. Despite the successes and the hard work of researchers, patients, and clinicians, there is still more to be done -- only 5 percent of rare diseases have an approved treatment option.
Working with stakeholders across public and private sectors, including rare disease researchers and patients, the biopharmaceutical industry has made important advances in developing innovative treatments for patients. Currently, there are more than 560 medicines in development for rare disease and more than 770 medicines have been approved to treat rare diseases over the past 35 years.
Key to the development of innovative medicines for rare diseases are novel drug development approaches that hold the promise to speed patient access to medicines. For over 25 years, the biopharmaceutical industry and the U.S. Food and Drug Administration (FDA) have negotiated the Prescription Drug User Fee Act (PDUFA) program, creating drug review efficiencies designed to accelerate the development and availability of new medicines to patients, while providing scientific and regulatory predictability to support biopharmaceutical innovation.
Key initiatives in the most recent PDUFA iteration, PDUFA VI, were designed to support cutting-edge approaches to drug development, including:
- Patient-focused drug development (PFDD), which provides an opportunity to incorporate the meaningful feedback and experiences (such as patient reported outcome measures) of rare disease patients into the drug development and review processes when evaluating the benefits and risks of a new medicine;
- Innovative clinical trial designs and statistical approaches (such as adaptive study designs, Bayesian statistical methodologies and model-informed drug development), which equip researchers and regulators with important information on drug safety and effectiveness, even with small a patient population, or when a traditional clinical trial is not possible or ethical;
- New drug development tools accelerate the development of rare disease medicines by identifying accurate, early measures of effectiveness – such as biomarkers and novel surrogate endpoints – in limited patient populations;
- Real-World Evidence (RWE) (i.e., health information collected outside of traditional clinical trial settings, for example from data sources such as electronic health records, payer claims, and patient registries) can provide more timely evidence regarding drug safety and efficacy, thereby accelerating clinical development.
The biopharmaceutical industry remains committed to meeting the needs of rare disease patients around the world. For example, in 2018 alone, FDA approved 34 novel medicines to help patients with rare diseases.
To learn more about rare disease research and the innovative medicines in development to treat rare diseases, visit: https://www.phrma.org/media/progress-in-fighting-rare-diseases