The Science of Hope: Understanding biomarkers
A well-defined regulatory pathway for biomarker qualification can help improve our understanding of drug development.
The Science of Hope: Understanding biomarkers.
A well-defined regulatory pathway for biomarker qualification can help improve our understanding of drug development.

The Science of Hope: Understanding biomarkers.
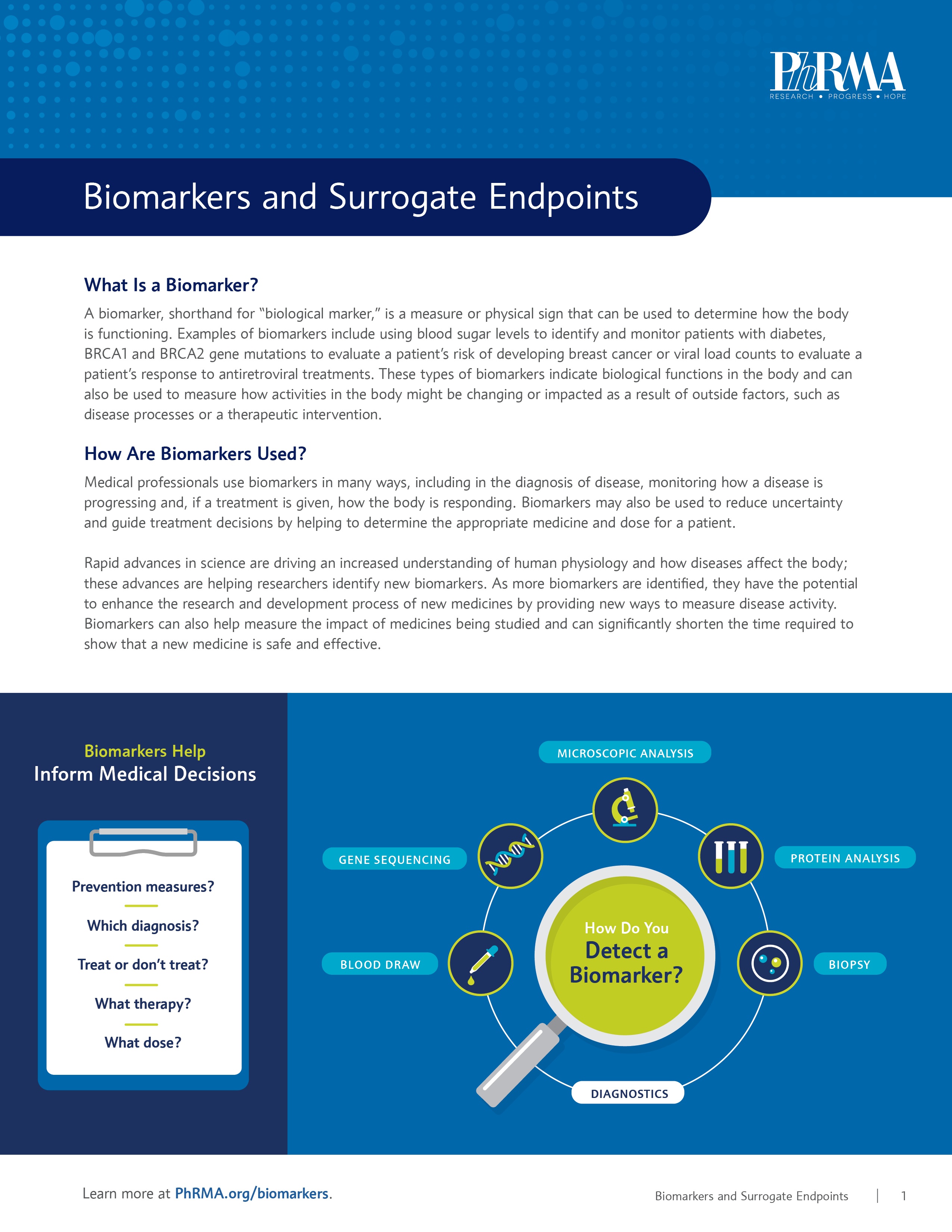
 In the age of personalized medicine, biomarkers (short for “biological markers”) are emerging as important tools in drug development. A biomarker is a measure or physical sign that can be used to determine how the body is functioning. This can help researchers develop more individualized treatments, provide an early warning sign for certain health risks and accelerate patient access to certain therapies.
In the age of personalized medicine, biomarkers (short for “biological markers”) are emerging as important tools in drug development. A biomarker is a measure or physical sign that can be used to determine how the body is functioning. This can help researchers develop more individualized treatments, provide an early warning sign for certain health risks and accelerate patient access to certain therapies.
Examples of biomarkers include using blood sugar levels to identify and monitor patients with diabetes, BRCA1/2 gene mutations to evaluate a patient’s risk of developing certain cancers, including breast and ovarian cancer, or viral load counts to evaluate a patient’s response to antiretroviral treatments.
As new biomarkers are identified, they have the potential to enhance the research and development process of new medicines by providing new ways to measure disease activity and the impact of the medicines being studied.
Biomarkers have many uses in the drug development process, including providing the ability to track the progress of disease. For disease areas where there is a significant unmet need for a serious disease or condition, like cancer, the U.S. Food and Drug Administration (FDA) may allow for the use of a surrogate endpoint to demonstrate the effect of a medicine. A surrogate endpoint is a biomarker (or other measure) that is expected to predict clinical benefit and can thus be substituted for a clinical endpoint.
For example, in clinical trials for cancer, researchers may be able to detect that a medicine is having an impact if there is a reduction in the size of a patient’s tumor before the disease fully manifests. The use of a biomarker as a surrogate endpoint can considerably shorten the time required prior to receiving FDA approval, giving patients access to medicines ahead of much longer confirmatory clinical trials.
 Beyond accelerating drug development as surrogate endpoints, the use of biomarkers can open new avenues for drug research. In neurodegenerative diseases, for example, biomarkers may provide researchers and clinicians with useful measurements that allow for early diagnosis before a patient suffers cognitive decline. Biomarkers may also allow researchers to develop treatments that quantitatively show that a treatment is slowing the progression of neurodegenerative diseases, which often result in a gradual decline in cognitive function.
Beyond accelerating drug development as surrogate endpoints, the use of biomarkers can open new avenues for drug research. In neurodegenerative diseases, for example, biomarkers may provide researchers and clinicians with useful measurements that allow for early diagnosis before a patient suffers cognitive decline. Biomarkers may also allow researchers to develop treatments that quantitatively show that a treatment is slowing the progression of neurodegenerative diseases, which often result in a gradual decline in cognitive function.
Given the rapid pace of the biomedical sciences and the growing role of biomarkers in accelerating drug development, PhRMA released a new fact sheet that explains how biomarkers are being used and detected in drug development and clinical trials to help get new medicines to patients sooner.
 In order to take advantage of the promise biomarkers hold in drug research, there is a need to establish a clearly defined biomarker qualification pathway at the FDA. A well-defined regulatory pathway for biomarker qualification can help improve our understanding of drug development, accelerate patient access to promising therapies, open new avenues for research and yield a more competitive and sustainable biopharmaceutical ecosystem.
In order to take advantage of the promise biomarkers hold in drug research, there is a need to establish a clearly defined biomarker qualification pathway at the FDA. A well-defined regulatory pathway for biomarker qualification can help improve our understanding of drug development, accelerate patient access to promising therapies, open new avenues for research and yield a more competitive and sustainable biopharmaceutical ecosystem.
Read more about proactive policy solutions to modernize the FDA and deliver innovative treatments to patients here.