It has been widely acknowledged that long patent examination delays cripple innovation, hinder economies, impede job creation and frustrate the launch of new products. Increased patent review times costs the global economy $10 billion annually, and is particularly harmful for small start-up firms. Every year of delay reduces the start-up’s employment growth by 21% and sales growth by 28% over the five years following the approval of the patent application. No country should accept the delayed launch of innovative technology or life-saving treatments because its patent office is plagued by long examination timelines and backlogs.
As the Office of the U.S. Trade Representative notes in its recent 2022 Special 301 report, which identifies countries that deny adequate and effective intellectual property protection or fair and equitable market access to American innovators, patent backlogs are a concern for the U.S. government with key trading partners, including Argentina, Brazil and Thailand. Indeed, the U.S. government flagged concerns with Brazil’s patent backlog as early as 1999.
Brazil, the largest economy in Latin America, has prioritized reducing the patent backlog, including through implementing the National Institute of Industrial Property’s “Plan to Tackle Patent Backlog,” hiring new examiners and leveraging work sharing programs. Moreover, the recent elimination of the Brazilian Health Regulatory Agency’s role with respect to the examination of pharmaceutical patent applications should help reduce the patent backlog.
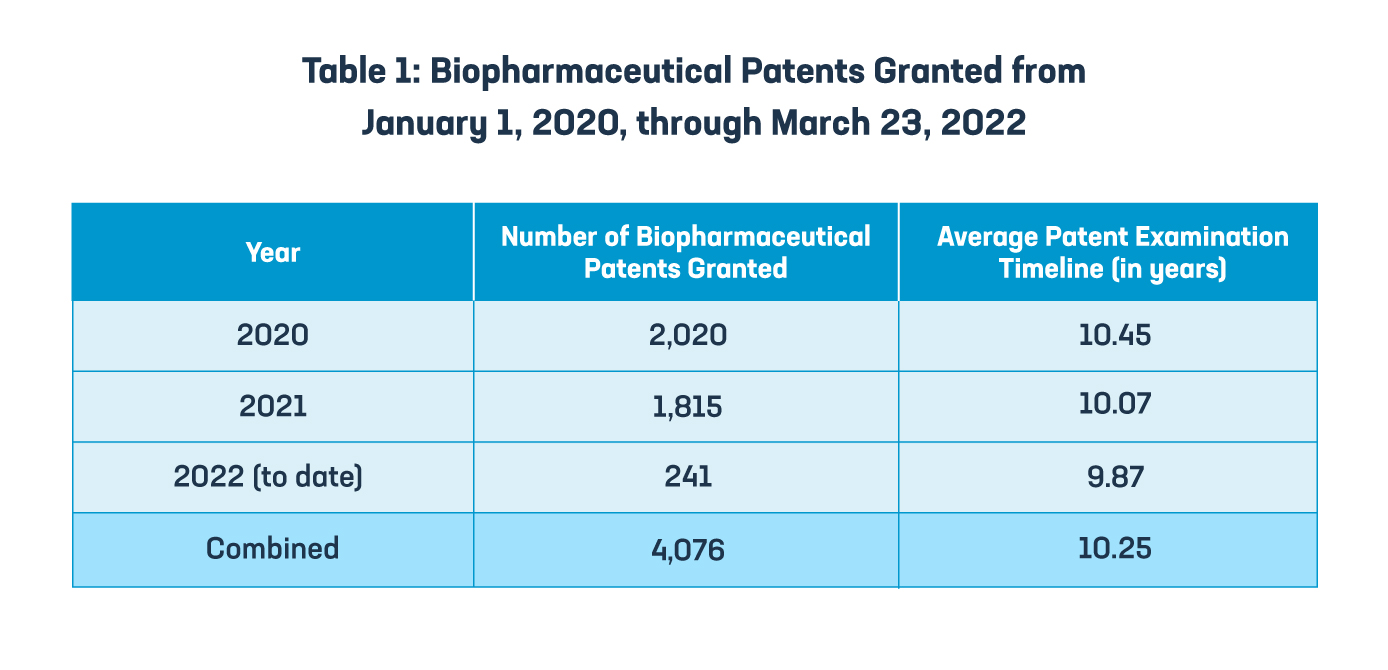
However, a recent analysis from Osha Bergman Watanabe & Burton LLP of the patent examination timelines for biopharmaceutical patents indicates that Brazil continues to suffer from long patent pendency timelines. As the data shows, the average patent examination timeline for biopharmaceutical patents granted between January 1, 2020, and March 23, 2022, is 10.25 years. Making matters worse, the Brazilian Supreme Court’s 2021 decision eliminating the sole paragraph of Article 40 of the Patent Law, which ensured a minimum patent term of 10 years from the date of patent grant in Brazil, leaves patent applicants across all technology sectors without a recognized mechanism to be compensated for unreasonable patent office examination delays.
Durable and comprehensive reform is needed to address Brazil’s patent examination backlog. A number of countries, recognizing the deleterious impact of patent office delays on the value of patents and their ability to facilitate access to medicines, have implemented mechanisms to safeguard against unreasonable delays during the examination of patent applications (commonly referred to as Patent Term Adjustment or PTA). Indeed, the United States has ensured that PTA provisions are in its recent free trade agreements, including the United States-Mexico-Canada Agreement.
Simply put, the time is now for Brazil to establish a PTA mechanism to ensure that innovators are not harmed by undue delays in the patent examination process, consistent with international best practices. The need for PTA is even more acute given that, based on a PhRMA analysis, Brazilian patients have access to significantly fewer new medicines when compared to OECD countries, and experience much longer than average wait times for those medicines that are launched in the market.




