Medicare Monday: Part D then and now
A comparison of what was said in the early years after Medicare Part D was created and what actually happened for costs and beneficiary premiums.
Medicare Monday: Part D then and now.
A comparison of what was said in the early years after Medicare Part D was created and what actually happened for costs and beneficiary premiums.

Medicare Monday: Part D then and now.
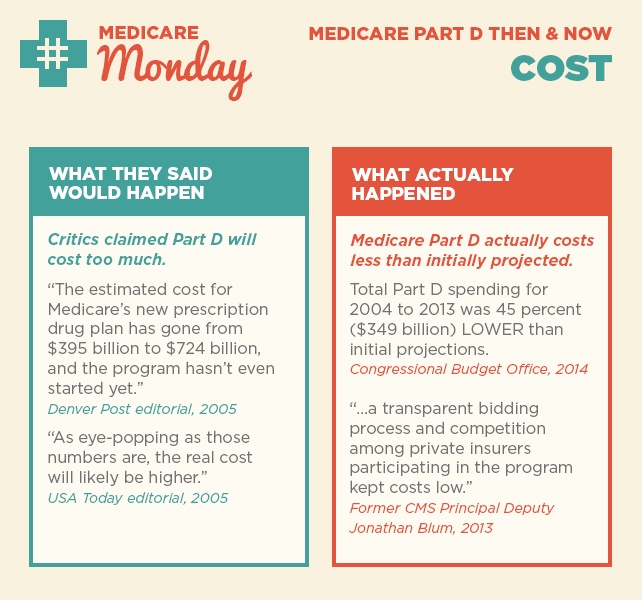
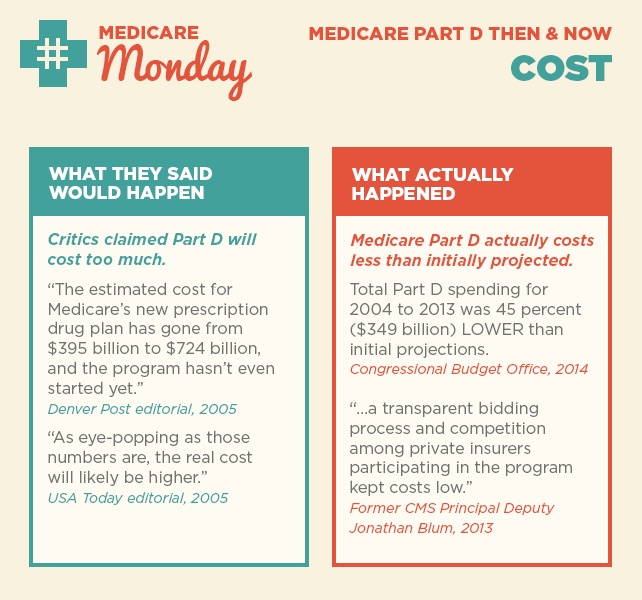
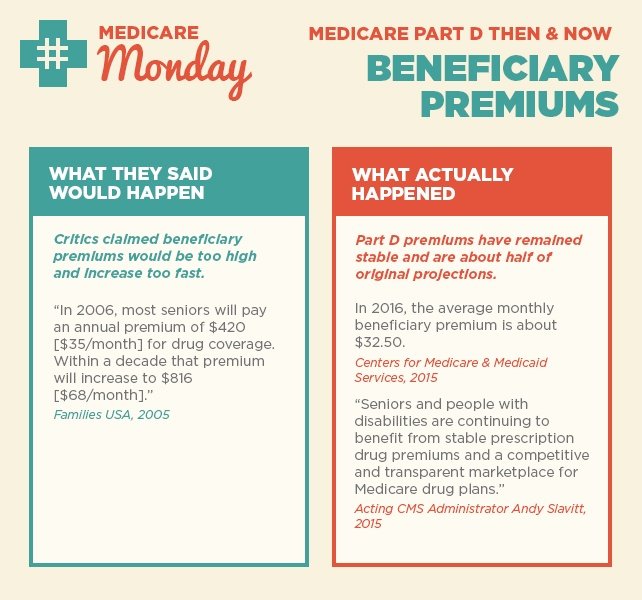
As we reflect on 10 years since the implementation of Medicare Part D, we’re taking a closer look at what was said in the early years after the law was passed and what actually happened. Over the next few weeks, follow along for our then-and-now series on Medicare Part D success.
Today we’re looking at two claims around Part D costs and beneficiary premiums.
Tune in next week for our next Part D Then and Now.