Today PhRMA released a new report that analyzes the share of new medicines that are available in over 70 countries and how long patients wait to receive them.
The Global Access to New Medicines Report includes comprehensive comparisons across countries in the G20, Organization for Economic Co-operation and Development (OECD) and several geographic regions.
A record number of new medicines are launching globally, but patients in most countries — including many developed countries — can access only a small share. On average, patients in OECD countries have access to only 29% of new medicines through a government health plan, while patients in the United States have access to 85%. In addition, patients in OECD countries wait an average of 41 months longer than patients in the United States for their government health plan to cover new medicines.
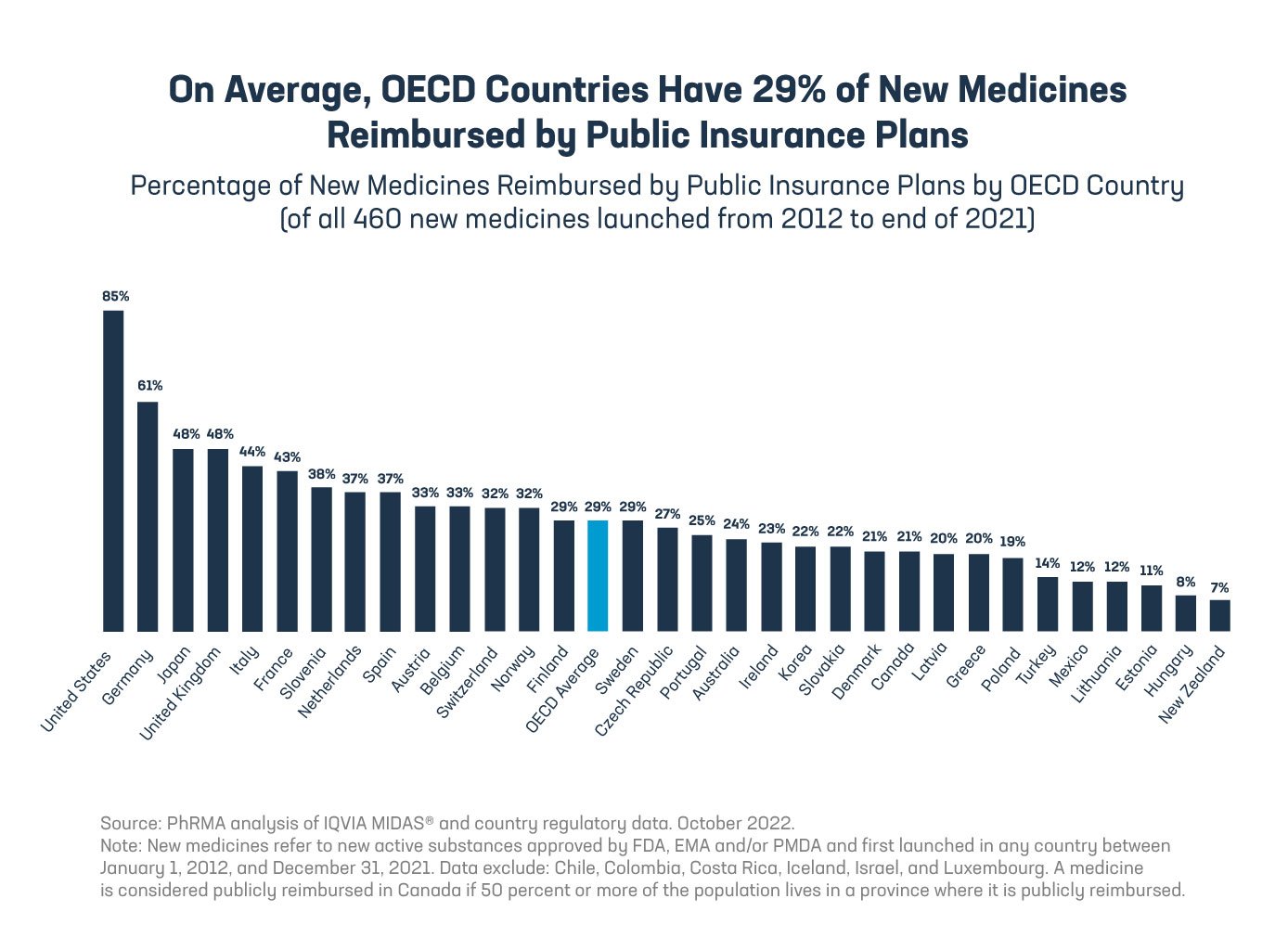
The report shines a light on the impact of policies that let governments act as gatekeepers between patients and new treatments. Most of the delay in patient access across OECD countries occurs after a new medicine has been approved as safe and effective by the local regulatory authority and often even after the product has been shipped by the manufacturer. For example, in Canada, 45% of new medicines were approved and launched by manufacturers 17 months after global first launch, but most patients in Canada wait nearly three more years for those medicines to be covered by their local government plan.
The report also shows that the United States is the world’s engine of biopharmaceutical innovation, with the most global first launches, the highest share of new medicines available and the fastest access for patients. However, provisions of the Inflation Reduction Act that impose government price setting threaten to reduce the number of new medicines being developed for patients around the world and could jeopardize access globally and in the United States.
Policymakers in all countries can enable timely patient access to new medicines by having strong intellectual property rights and enforcement, science-based and globally-harmonized regulatory review, and transparent pricing and reimbursement policies that value innovation. The perils of anti-innovation policies are catalogued in PhRMA’s recent submissions to the U.S. Trade Representative for the annual National Trade Estimate Report and Special 301 Report.
Please click here to download the full Global Access to New Medicines Report.




