Medicines have significantly increased chances of cancer survival around the globe
Since a peak in 1991, cancer mortality rates have declined by

Medicines have significantly increased chances of cancer survival around the globe.
Since a peak in 1991, cancer mortality rates have declined by

Medicines have significantly increased chances of cancer survival around the globe.
Since a peak in 1991, cancer mortality rates have declined by as much as 27% around the world. These gains in cancer survival are largely attributed to new and innovative therapies. According to the CDC, for patients with childhood leukemia, prostate, and breast cancers, five-year survival has increased steadily in most developed countries, with some countries reporting five-year survival rates of more than 85%.
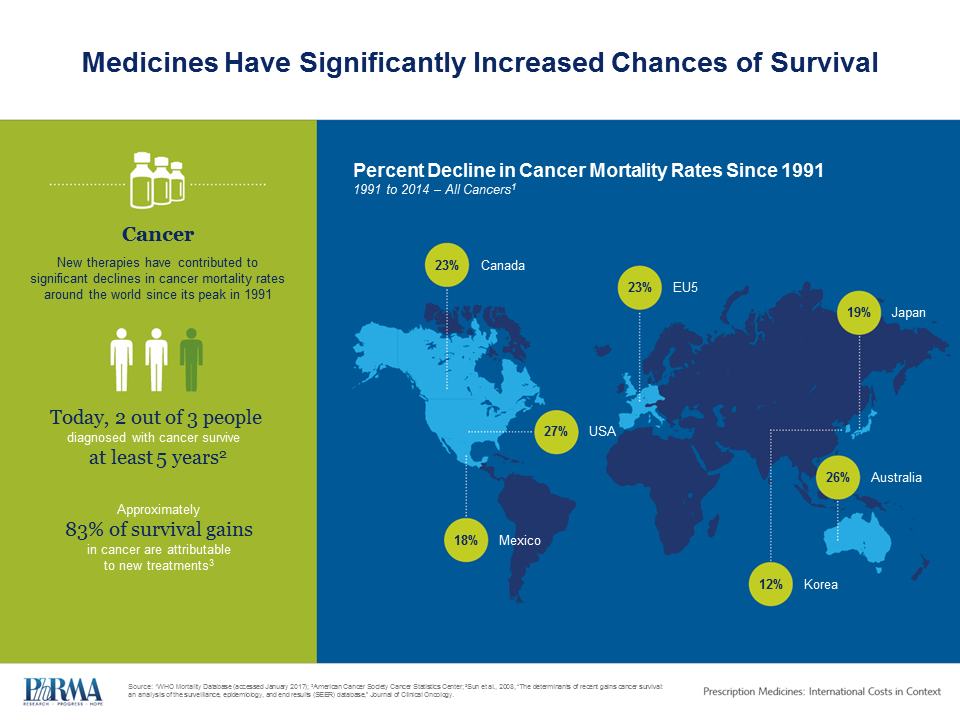
New treatments continue to alter the course of previously fatal diseases and there are now more promising cancer medicines in the pipeline than at any time in history. Personalized medicines such as immunotherapy are transforming cancer care in ways never before seen, using patients’ immune systems to fight or eliminate cancers.
To learn more check out our International Cost in Context information.