With the average cost of developing, testing and receiving approval for a new medicine at $2.6 billion and rising, biopharmaceutical companies are continuously looking at new ways to increase efficiencies in their research and development capabilities.
In a new report, “Profiles of New Approaches to Improving the Efficiency and Performance of Pharmaceutical Drug Development," the Tufts Center for the Study of Drug Development found that biopharmaceutical companies are using a variety of innovative approaches to improve the efficiency and productivity of their research efforts.
Key findings include:
- New approaches to target validation to improve the ability to define the role of proteins, genes and other molecules involved in disease pathogenesis, which can increase the chances that medicines will act on these targets.
- Innovative trial designs and methodologies which hold immense potential for improving success rates and creating greater efficiencies in conducting clinical trials.
- New drug development tools, such as patient-reported outcomes or biomarkers, have the potential to improve efficiency at many points in the research and development process.
- Improvements in IT structure to enhance data utilization can provide invaluable information on the benefits and risks of a medicine to help speed development.
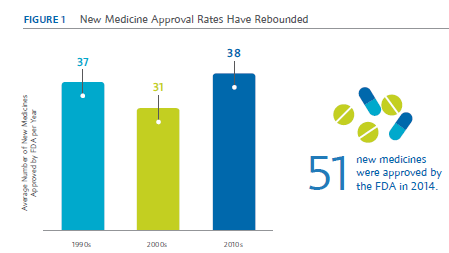
New research from Boston Consulting Group (BCG) suggests these efforts may already be contributing to the record number of new drug approvals in 2014. In 2014 alone, the Food and Drug Administration (FDA) approved 51 new medicines, the highest number since 1996.
Due to these and other innovations, including in the manufacturing process, biopharmaceutical companies continue to see increased productivity. Despite the challenges in the research and development landscape, there are currently more than 7,000 new medicines in development. To learn more about the exciting new innovations in biopharmaceutical research and development, read the full report.





