Research doesn’t end once a medicine is first approved by the U.S. Food and Drug Administration (FDA). Researchers often spend years after an initial approval exploring and obtaining approval of new indications for medicines to treat other patient populations desperate for new treatment options. Patients with cancer or rare diseases often depend on this post-approval research.
But now, under the Inflation Reduction Act’s (IRA) price setting provisions, small molecule medicines can be selected for price setting as early as seven years after the FDA’s initial approval, which is long before the end of their average 13-to-14-year effective patent life. As a result, this “pill penalty” may discourage companies from researching and developing small molecule medicines at all. Additionally, because these medicines can be selected so early, companies are also discouraged from doing R&D in the years following a medicine’s initial FDA approval — putting at risk the progress we’ve made to fight cancer and other diseases.
Analysis of small molecule prescription medicines receiving initial FDA approval between January 1, 2006, and December 31, 2012, by Partnership for Health Analytic Research (PHAR) finds the IRA could acutely discourage critical post-approval research and development — hindering innovation for cancer, rare diseases and other treatment advances.
The analysis found:
Of all the small molecule medicines in this study, more than half received at least one additional indication after the initial FDA approval.
45% of these post-approval indications were approved seven or more years after initial approval. These later-approved indications are the ones most at risk from the IRA’s shortened timeframe.
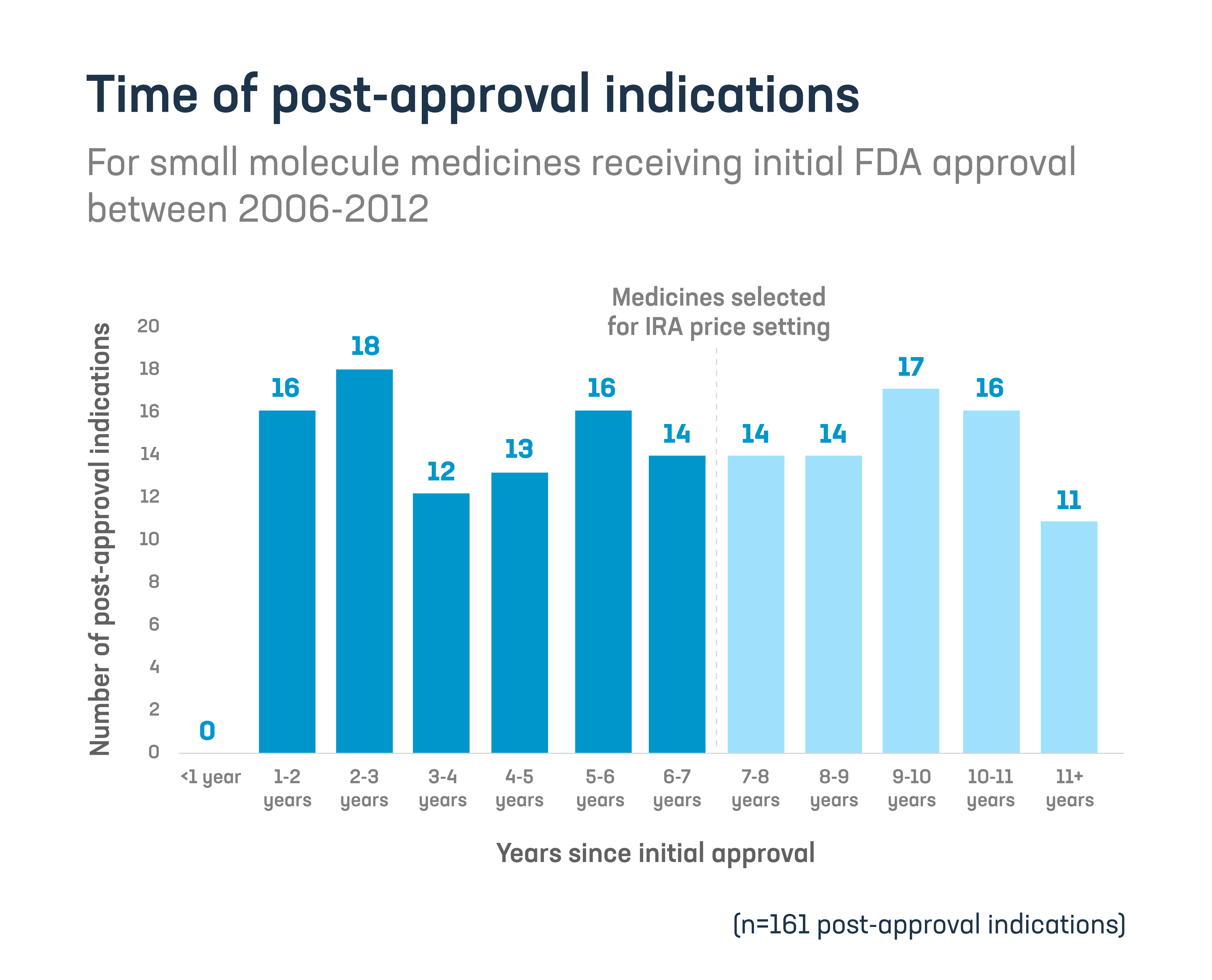
Post-approval indications represent important advances for patients and are particularly common for rare diseases and cancer treatments, as researchers learn more and follow scientific leads following initial approval.
- 72% of post-approval indications expanded a medicine’s use to new age groups or other defined populations, and 25% were for new disease targets.
- 61% of small molecule cancer medicines were awarded at least one post-approval indication.
- 63% of medicines first approved as orphan drugs were awarded at least one post-approval indication.
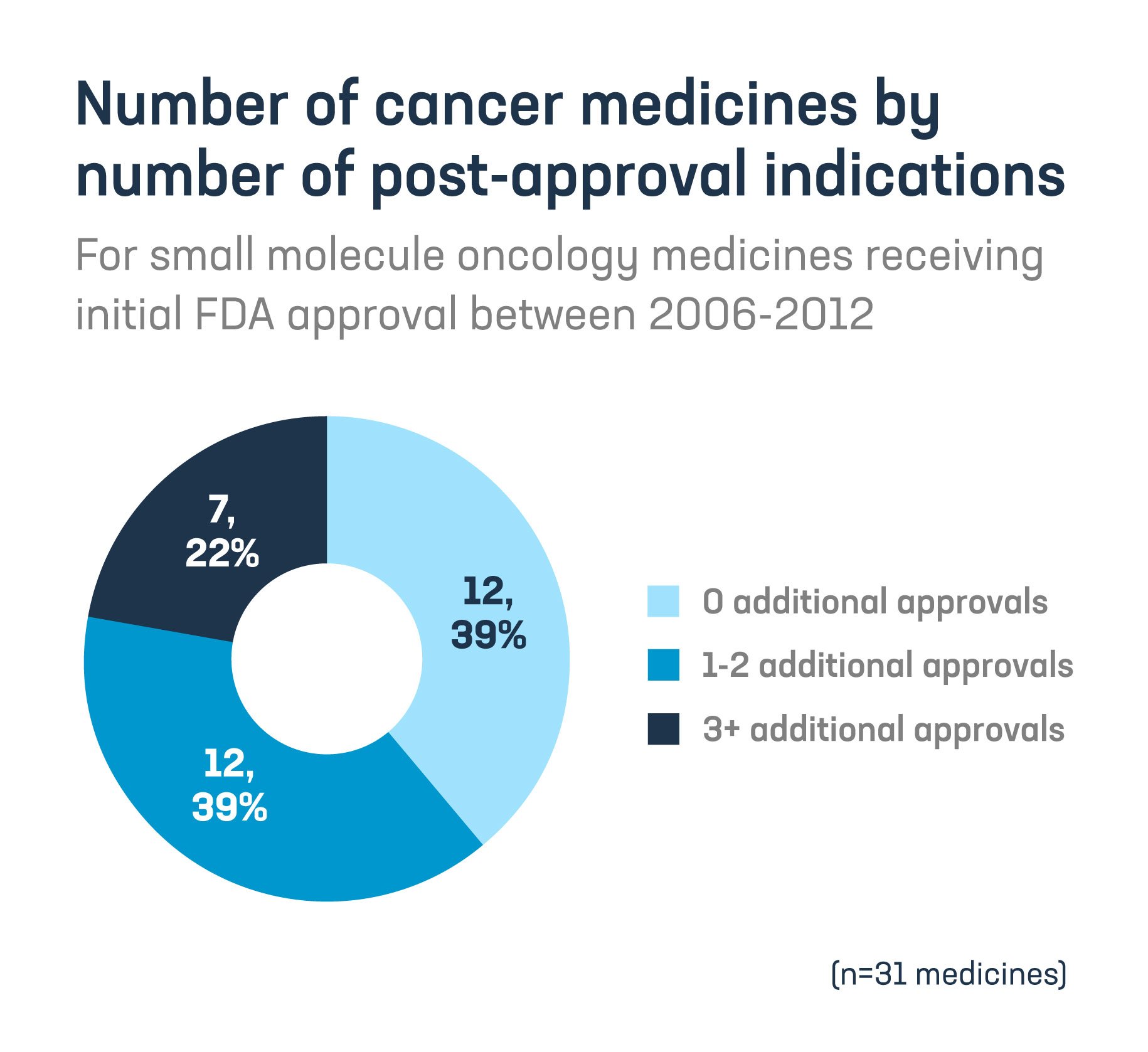
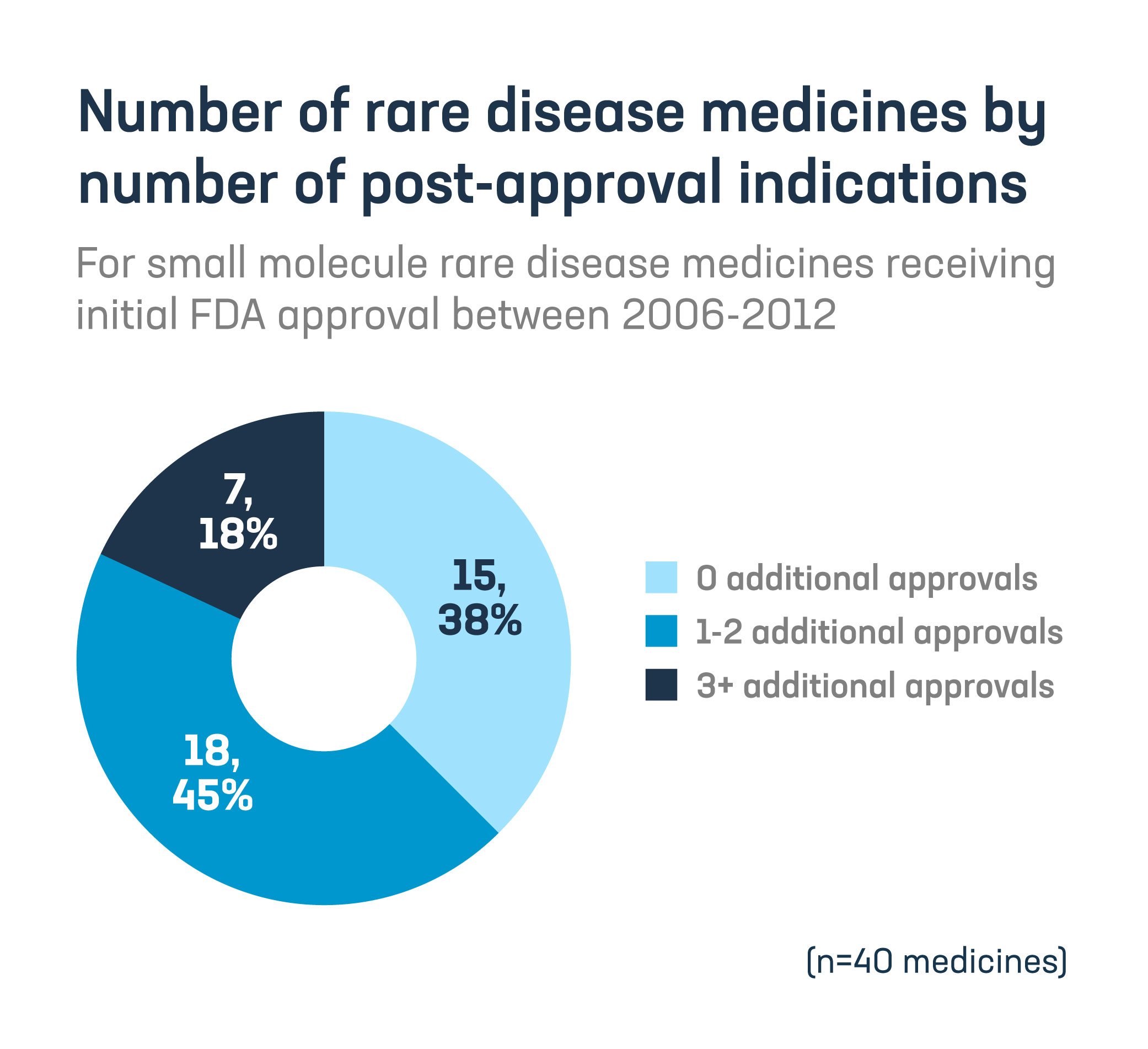
Congress must prioritize reforms to fix the “pill penalty” and protect innovation for lifesaving care.
For more information on the impact price-setting policies have on cancer and rare disease innovation, read more of PHAR’s analysis here. To see the impact directly on cancer, explore our fact sheet.






