Despite what critics say, the U.S. biopharmaceutical industry spends three times more on the research and development (R&D) of new treatments and cures than on the marketing and promotion of medicines. Misleading claims on how much is spent on marketing and promotion perpetuate a narrative riddled with inaccurate data and information and results in policy proposals that can harm patients.
Here is the truth about the marketing and promotion of medicines.
- The biopharmaceutical industry spends three times more on R&D than marketing and promotion. According to the Journal of the American Medical Association (JAMA), U.S. biopharmaceutical companies spent $90.5 billion in 2016 on R&D, over three times more than the $28.1 billion spent on marketing and promotion that year.
![PhRMA_R&D-Chart_Gif-2[2]](/-/media/Project/PhRMA/PhRMA-Org/HubspotImages/hubfs/464546/PhRMA_R-D-Chart_Gif-2-2.jpg)
- Marketing and promotion strengthen investment in new treatments and cures. Some critics claim every dollar spent on marketing is a dollar less that can be invested in R&D, but the economics behind the biopharmaceutical sector don’t work that way. In fact, a survey of PhRMA members shows that in the past two decades, R&D investment grew significantly during the same time that direct-to-consumer (DTC) advertising began.
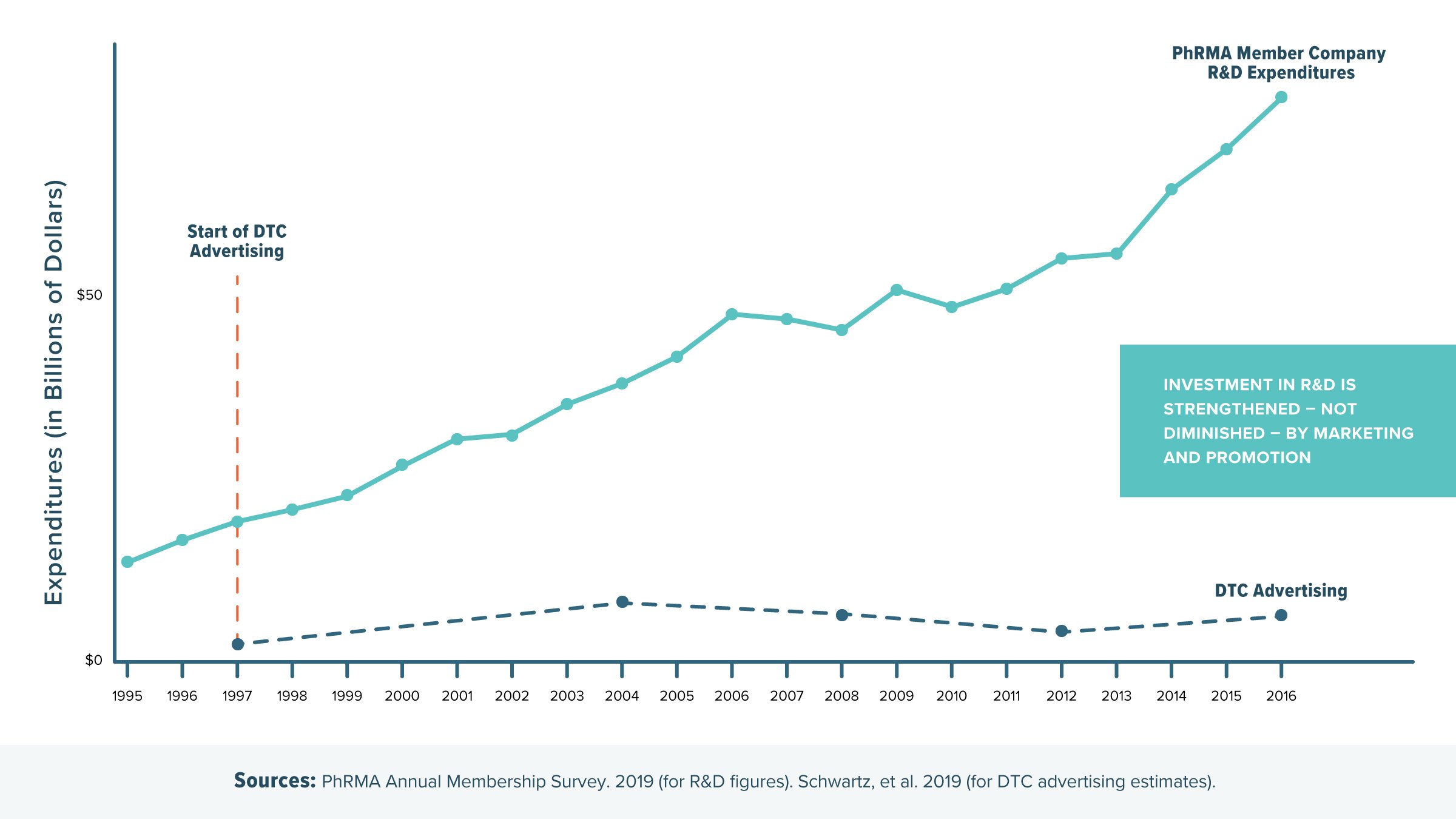
- Marketing and promotion benefit patients, providers and the health care system. DTC advertising raises disease awareness, removes stigma and promotes medicine adherence. Plus, biopharmaceutical marketing and promotion includes sharing information directly with health care professionals to keep them updated about the latest information, including findings from new clinical studies and about new dosing information.
- The FDA heavily regulates medicine marketing and promotion. The FDA makes sure biopharmaceutical marketing and promotion materials are accurate, non-misleading and include a “fair balance” of a medicine’s benefits and risks. The PhRMA Guiding Principles on Direct to Consumer Advertisements about Prescription Medicines also provide guidelines for companies’ promotional communications.
To learn more, download the new issue brief and one pager.



![PhRMA_R&D-Chart_Gif-2[2]](/-/media/Project/PhRMA/PhRMA-Org/HubspotImages/hubfs/464546/PhRMA_R-D-Chart_Gif-2-2.jpg)

