It’s hard to believe, but – this year alone – more than 162,000 Americans will be diagnosed with a form of blood cancer, such as leukemia, lymphoma or myeloma. Fortunately, science has advanced quickly in recent years, opening doors for more precise treatments for more patients. This progress is certainly encouraging, but more work remains to find effective, targeted medicines for all patients impacted by blood cancer.
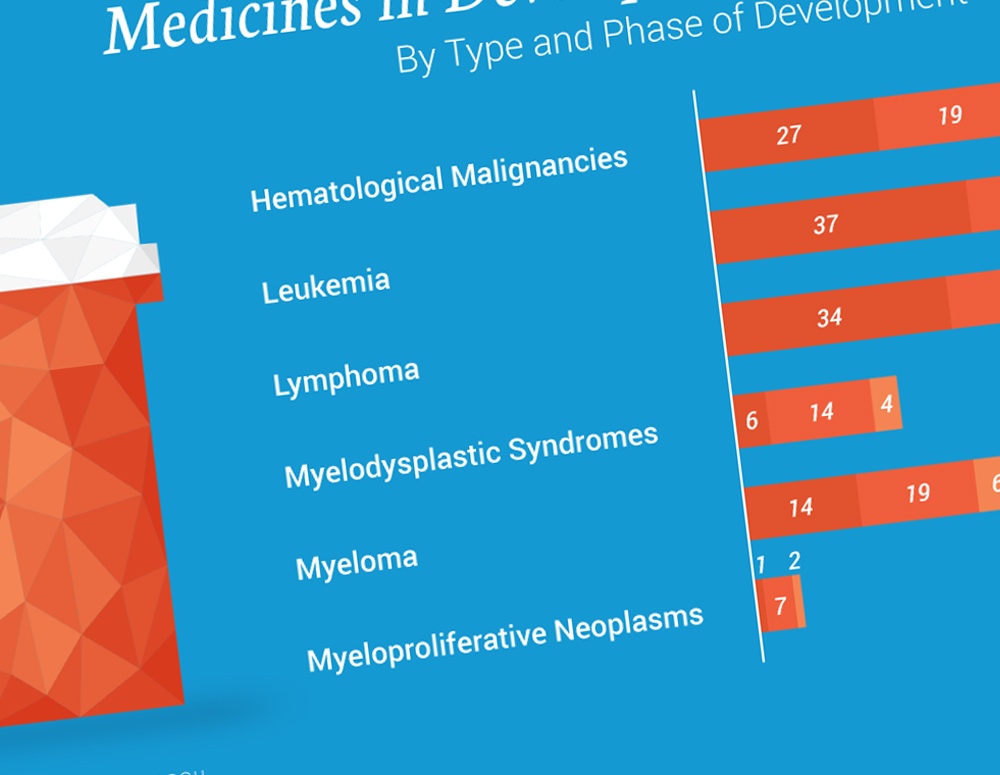
According to a new report from PhRMA and The Leukemia & Lymphoma Society (LLS), biopharmaceutical companies – working alongside nonprofit organizations, academic institutions, government researchers and other partners – currently have more than 240 new medicines in every phase of development to treat blood cancers. A breakdown of these investigational medicines is captured in the infographic below:
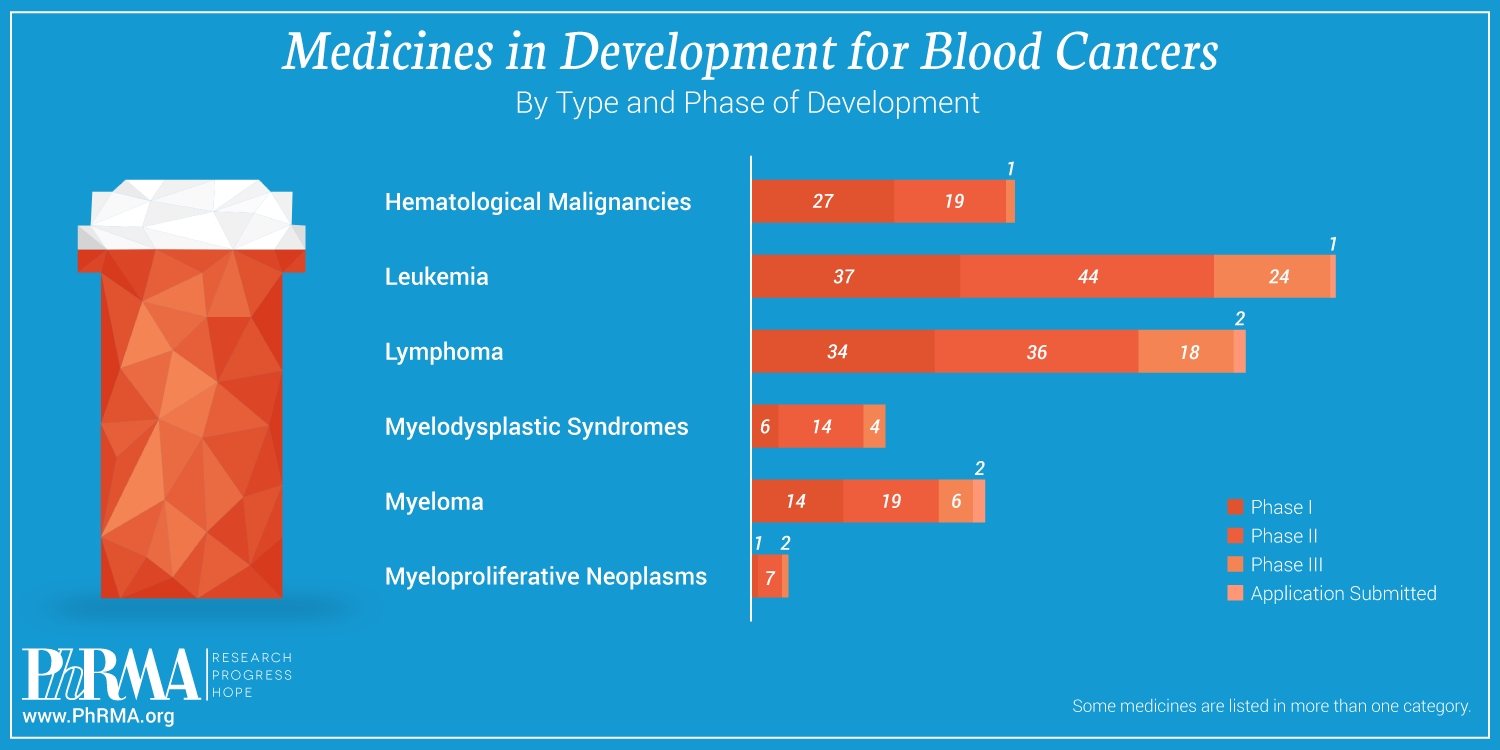
As biopharmaceutical researchers and scientists continue to learn more about the intricacies behind blood cancers and the best ways to treat them, they have begun to unlock the potential of medicines to work at a molecular level to target and fight the 35 types of leukemia and the 50 types of lymphomas that have been identified in recent years.
Additional Resources
And the really encouraging news for patients? These efforts are working in practice.
Blood cancer survival rates are on the rise across the board. The overall five-year relative survival rate for leukemia patients has more than quadrupled since 1960, from 14 percent to 60 percent. And for patients diagnosed with Hodgkin lymphoma at age 45 or younger, the five-year survival rate has increased over the years to nearly 94 percent.
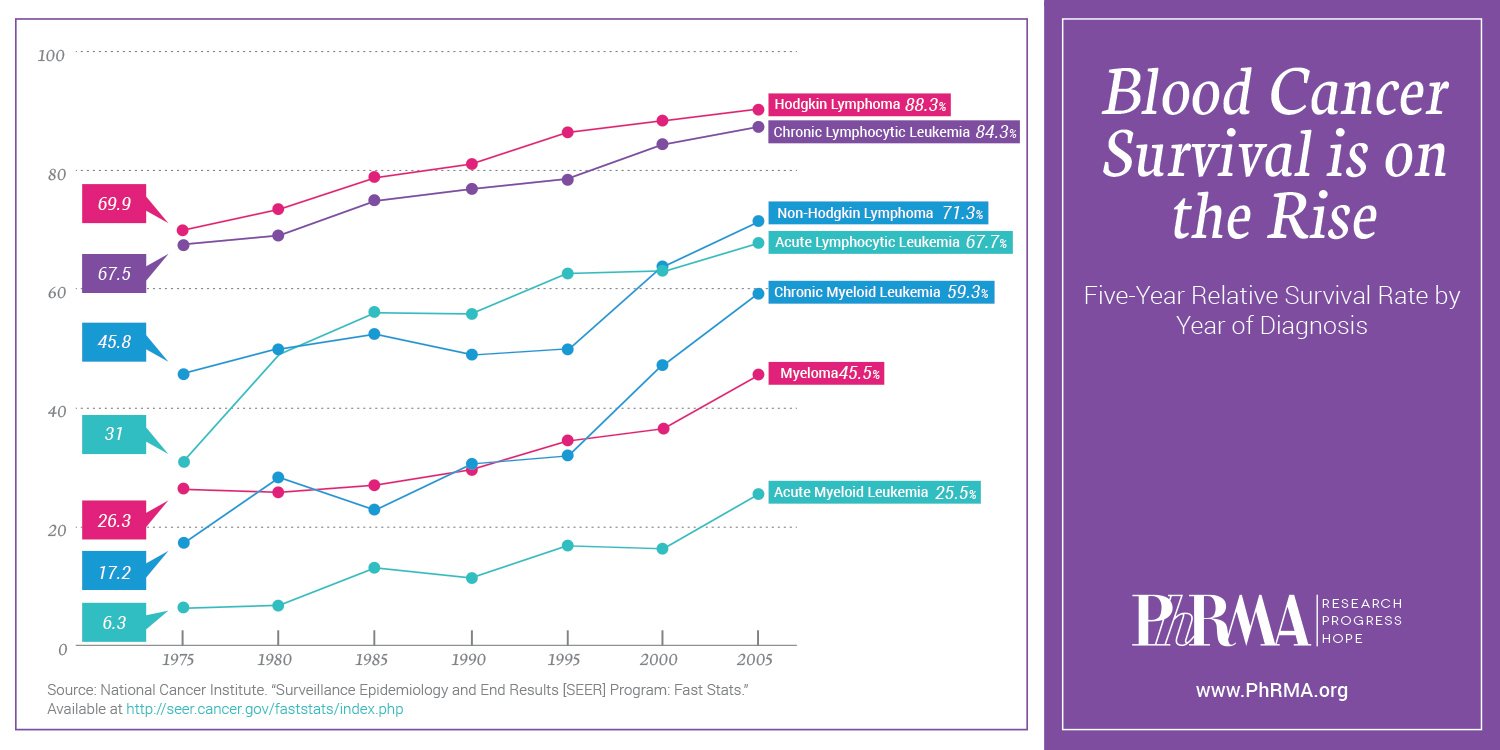
Despite this significant progress, there is more work to do. That’s why PhRMA takes great pride in partnering with organizations like The Leukemia & Lymphoma Society to help fund research into more effective medicines and treatments that extend and improve patients’ lives. Such collaboration is critical as we work to move biopharmaceutical research forward together – and strive to lessen the burden of leukemia, lymphoma, Hodgkin lymphoma and myeloma for patients and their families.