September is National Sickle Cell Awareness Month and a time for us to underscore the biopharmaceutical industry’s ongoing commitment to improve the lives of patients, families and communities affected by sickle cell disease.
Sickle cell disease (SCD) is an inherited rare disease impacting approximately 100,000 Americans. SCD is caused by a mutation in the gene that tells the body how to make hemoglobin — the protein in red blood cells that carries oxygen. Normal red blood cells are flexible and round. But in SCD patients, red blood cells are rigid and crescent-shaped, which means they are more likely to get caught in small blood vessels and reduce the flow of oxygen to certain parts of the body. Patients suffering from SCD often experience significant and severe chronic pain and require life-long management with medications and regular blood transfusions to help decrease the episodes of pain, relieve symptoms and prevent complications. The disease typically manifests in children before one year of age and life expectancy averages 54 years for patients in the United States.
SCD disproportionately affects people of Black or African American descent who make up 85% of the SCD patient population in the United States. About one in 365 Black or African American babies are born with SCD and about one in 13 Black or African American babies are born with sickle cell trait. Throughout their lifetime, these patients will often experience significant pain that has a severe impact on their quality of life. For example, more than half of SCD patients report the disease affecting their employment status as the chronic pain they experience may force them to reduce their working hours or stop working completely. These patients are expected to earn $750,000 less over the course of their lifetime.
As we recognize National Sickle Cell Awareness Month, we must reinforce the need to address health disparities and improve the quality of life for those suffering from the disease. Early detection, better education for providers and expanded access to treatment options will help patients better mitigate symptoms, manage health care costs and enforce equitable access to care. Patients also need novel treatments that would transform SCD care. Currently, the only cure for SCD is a bone marrow or stem cell transplant. Transplants are considered for younger patients with severe SCD, but they require a matching donor and can have serious risks.
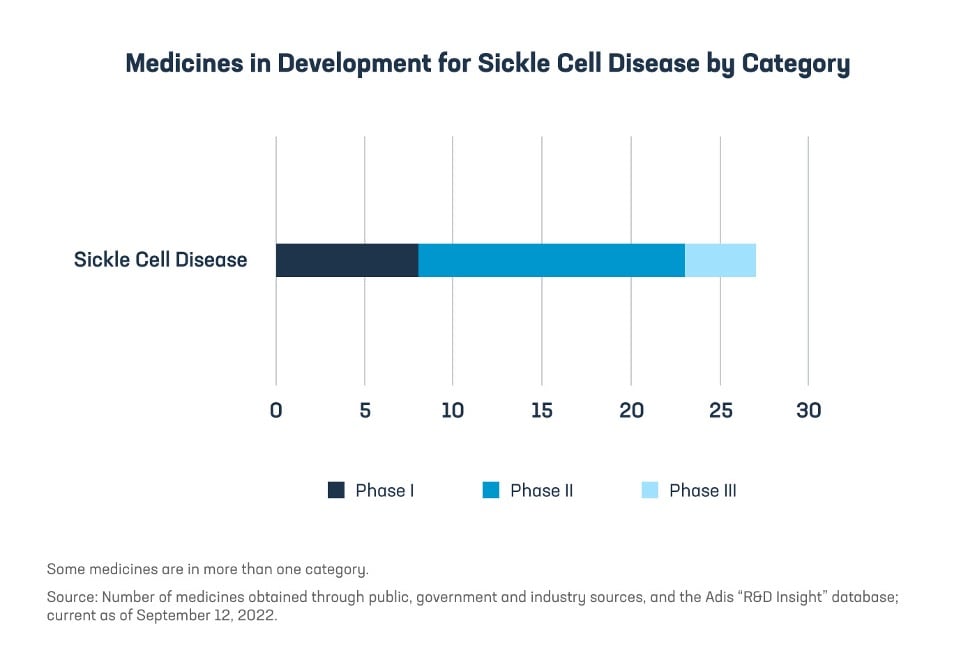
Fortunately, because of the continued investments and efforts by the biopharmaceutical industry, the future is more promising than ever before with 27 medicines currently in development for the treatment of SCD. Among these are gene therapies which represent the latest in scientific treatment advances and explore new ways to target SCD through cutting-edge technologies like RNA interference, CRISPR, and lentivirus for stem cell-based therapy and gene therapy.
With their unique approach to treating SCD, one of these potential gene therapies may offer long term or even curative benefits to patients with a single administration. In fact, gene therapies in the late stages of development have demonstrated an almost complete reduction in pain crisis in SCD patients in the years following administration. As a result, these therapies hold promise in dramatically improving quality of life of SCD patients, including the Black and African American individuals who are disproportionately impacted by this inherited illness. By this improvement in quality of life and significant reduction in pain crises, individuals with SCD may be able to live longer, healthier lives and maintain more consistent and reliable employment.
The significant medical advancements in the treatment of SCD to date demonstrate the necessity of preserving a biopharmaceutical ecosystem that fosters future innovations and continues to focus on developing new medicines for patients. Furthermore, we must ensure that those who may benefit from gene therapies will have access to them. The full value of new treatments like gene therapies can only be realized over a patient’s lifetime, which is why private payers are exploring innovative contracting approaches that account for the long-term value these novel medicines provide.
Learn more about the medicines in development for sickle cell disease and other blood disorders by reading our latest report.